Extracellular vesicle-mediated cell-cell communication in haematological neoplasms
- PMID: 29158313
- PMCID: PMC5717438
- DOI: 10.1098/rstb.2016.0484
Extracellular vesicle-mediated cell-cell communication in haematological neoplasms
Abstract
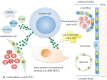
Crosstalk between bone marrow tumour cells and surrounding cells, including bone marrow mesenchymal stromal cells (BM-MSCs), endothelial cells and immune cells, is important for tumour growth in haematological neoplasms. In addition to conventional signalling pathways, extracellular vesicles (EVs), which are endosome-derived vesicles containing proteins, mRNAs, lipids and miRNAs, can facilitate modulation of the bone marrow microenvironment without directly contacting non-tumourous cells. In this review, we discuss the current understanding of EV-mediated cell-cell communication in haematological neoplasms, particularly leukaemia and multiple myeloma. We highlight the actions of tumour and BM-MSC EVs in multiple myeloma. The origin of EVs, their tropism and mechanism of EV transfer are emerging issues that need to be addressed in EV-mediated cell-cell communication in haematological neoplasms.This article is part of the discussion meeting issue 'Extracellular vesicles and the tumour microenvironment'.
Keywords: bone marrow mesenchymal stromal cells; extracellular vesicles; hypoxia; leukaemia; miRNA; multiple myeloma.
© 2017 The Author(s).
Conflict of interest statement
We have no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
