Krüpple-like-factor 4 Attenuates Lung Fibrosis via Inhibiting Epithelial-mesenchymal Transition
- PMID: 29158503
- PMCID: PMC5696468
- DOI: 10.1038/s41598-017-14602-7
Krüpple-like-factor 4 Attenuates Lung Fibrosis via Inhibiting Epithelial-mesenchymal Transition
Abstract
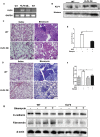
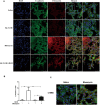
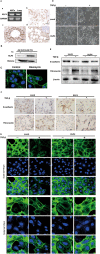
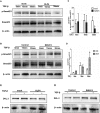
Epithelial-mesenchymal transition (EMT) plays an important role in the pathogenesis of idiopathic pulmonary fibrosis (IPF). Krüpple-like-factor 4 (KLF4), has been suggested to play an important role in the phenotype transition. However, its function in pulmonary fibrosis and EMT of human alveolar epithelial cells (AECs) remains unclear. This study aimed to examine the role of KLF4 in pulmonary fibrosis and EMT. Decreased expression of KLF4 was first observed in human IPF lung tissues and models of bleomycin-induced pulmonary fibrosis. Transgenic mice with overexpression of KLF4 were subjected to bleomycin-induced pulmonary fibrosis model and showed attenuated lung fibrosis and EMT compared to wild type group. Furthermore, the effects overexpression and knockdown of KLF4 on TGF-β1-induced EMT were examined in AECs. Adenovirus-mediated overexpression of KLF4 attenuated TGF-β1-induced EMT and activation of Smad2/3 and Dvl in AECs. Conversely, knockdown of KLF4 promoted the activation of pathways above mentioned and TGF-β1-induced EMT. Our results demonstrates that KLF4 plays an important role in bleomycin-induced lung fibrosis through suppressing TGFβ1-induced EMT. Thus, it may serve as a potential target for the treatment of pulmonary fibrosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Inhibition of mTOR ameliorates bleomycin-induced pulmonary fibrosis by regulating epithelial-mesenchymal transition.Biochem Biophys Res Commun. 2018 Jun 12;500(4):839-845. doi: 10.1016/j.bbrc.2018.04.148. Epub 2018 May 2. Biochem Biophys Res Commun. 2018. PMID: 29704504
-
Tetraspanin 1 as a mediator of fibrosis inhibits EMT process and Smad2/3 and beta-catenin pathway in human pulmonary fibrosis.J Cell Mol Med. 2019 May;23(5):3583-3596. doi: 10.1111/jcmm.14258. Epub 2019 Mar 14. J Cell Mol Med. 2019. PMID: 30869194 Free PMC article.
-
Astragaloside IV modulates TGF-β1-dependent epithelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis.J Cell Mol Med. 2018 Sep;22(9):4354-4365. doi: 10.1111/jcmm.13725. Epub 2018 Jul 4. J Cell Mol Med. 2018. PMID: 29971947 Free PMC article.
-
Radiation-Induced Pulmonary Epithelial-Mesenchymal Transition: A Review on Targeting Molecular Pathways and Mediators.Curr Drug Targets. 2018;19(10):1191-1204. doi: 10.2174/1389450119666180207092234. Curr Drug Targets. 2018. PMID: 29412104 Review.
-
Cytokines as drivers: Unraveling the mechanisms of epithelial-mesenchymal transition in COVID-19 lung fibrosis.Biochem Biophys Res Commun. 2023 Dec 17;686:149118. doi: 10.1016/j.bbrc.2023.10.050. Epub 2023 Oct 14. Biochem Biophys Res Commun. 2023. PMID: 37931361 Review.
Cited by
-
Human alveolar epithelial cells type II are capable of TGFβ-dependent epithelial-mesenchymal-transition and collagen-synthesis.Respir Res. 2018 Jul 24;19(1):138. doi: 10.1186/s12931-018-0841-9. Respir Res. 2018. PMID: 30041633 Free PMC article.
-
S-nitrosation impairs KLF4 activity and instigates endothelial dysfunction in pulmonary arterial hypertension.Redox Biol. 2019 Feb;21:101099. doi: 10.1016/j.redox.2019.101099. Epub 2019 Jan 7. Redox Biol. 2019. PMID: 30660098 Free PMC article.
-
Epigallocatechin-3-Gallate (EGCG), an Active Compound of Green Tea Attenuates Acute Lung Injury Regulating Macrophage Polarization and Krüpple-Like-Factor 4 (KLF4) Expression.Molecules. 2020 Jun 20;25(12):2853. doi: 10.3390/molecules25122853. Molecules. 2020. PMID: 32575718 Free PMC article.
-
KLF4 Alleviates Hypertrophic Scar Fibrosis by Directly Activating BMP4 Transcription.Int J Biol Sci. 2022 May 9;18(8):3324-3336. doi: 10.7150/ijbs.71167. eCollection 2022. Int J Biol Sci. 2022. PMID: 35637963 Free PMC article.
-
Single-Cell RNA-Seq Identifies Dynamic Cardiac Transition Program from ADCs Induced by Leukemia Inhibitory Factor.Stem Cells. 2022 Oct 21;40(10):932-948. doi: 10.1093/stmcls/sxac048. Stem Cells. 2022. PMID: 35896368 Free PMC article.
References
-
- Selman M, Pardo A. Idiopathic pulmonary fibrosis: misunderstandings between epithelial cells and fibroblasts? Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:165–172. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

