Efficacy and safety of DFN-15, an oral liquid formulation of celecoxib, in adults with migraine: a multicenter, randomized, placebo-controlled, double-blind, crossover study
- PMID: 29158678
- PMCID: PMC5683772
- DOI: 10.2147/NDT.S151834
Efficacy and safety of DFN-15, an oral liquid formulation of celecoxib, in adults with migraine: a multicenter, randomized, placebo-controlled, double-blind, crossover study
Abstract
Background: The objective of this proof-of-concept study was to assess the safety, efficacy, and potential for dose response of a new oral liquid formulation of celecoxib, DFN-15, in adults with migraine. Variability in patient-identified most bothersome symptom (MBS) across 3 migraine attacks was also evaluated.
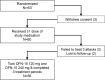
Methods: This was a randomized, placebo-controlled, double-blind, 3-treatment, 6-sequence, 3-period, crossover study of 3 treatments (DFN-15 120 mg, DFN-15 240 mg, and placebo) administered at the onset of moderate to severe headache.
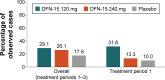
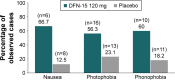
Results: Of 63 randomized subjects, 56 (89%) took single doses of DFN-15 120 mg and 240 mg and completed all 3 treatment periods. Most subjects were female (75.0%) and white (86.7%), with a mean age of 43.6 years. Both doses of DFN-15 achieved a higher 2-hour pain-free response than placebo (29.1% for 120 mg, 26.1% for 240 mg, and 17.6% for placebo), but the differences were not statistically significant. Photophobia was most commonly reported as the MBS, but for 53% of subjects (27/51), their identified MBS varied across the 3 studied attacks. The most common treatment-emergent adverse events with DFN-15 were dysgeusia (≤11.8%) and nausea (≤5.9%).
Conclusion: Both doses of DFN-15 outperformed placebo for the 2-hour pain-free end point, but due to a carryover effect with placebo, the differences were not statistically significant. Since response to both doses was similar, DFN-15 120 mg is being further developed for the management of acute migraine. Further study is needed to determine whether the current findings are altered by larger or different trial designs (ClinicalTrials.gov identifier: NCT02472418).
Keywords: acute treatment; celecoxib; efficacy; episodic migraine; safety.
Conflict of interest statement
Disclosure SM and AB are employed by and own stock in Dr Reddy’s Laboratories. The authors report no other conflicts of interest in this work.
Figures
References
-
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55(6):754–762. - PubMed
-
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the american headache society evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3–20. - PubMed
-
- Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284(10):1247–1255. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical