Autoimmune response to AGE modified human DNA: Implications in type 1 diabetes mellitus
- PMID: 29159085
- PMCID: PMC5685016
- DOI: 10.1016/j.jcte.2014.05.002
Autoimmune response to AGE modified human DNA: Implications in type 1 diabetes mellitus
Abstract
Aims: Non-enzymatic glycation of DNA both in vivo and in vitro results in generation of free radicals, known as glycoxidation. Glycoxidation leads to structural perturbation of DNA resulting in generation of neo-antigenic epitopes having implication in autoimmune disorders like diabetes mellitus. In this study human placental DNA was glycated with methylglyoxal (MG) and lysine (Lys) in the presence of Cu2+ and its auto-antibody binding was probed in Type 1 diabetes patients.
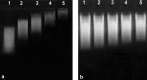
Methods: Glycation was carried out by incubating DNA with MG, Lys and Cu2+ for 24 h at 37 °C. Carboxyethyl deoxyguanosine (CEdG) formed in glycation reaction was studied by LC-MS and the pathway for Amadori formation was studied by ESI-MS techniques. Furthermore, binding characteristics of auto-antibodies in diabetes patients were assessed by direct binding, competitive ELISA and band shift assay.
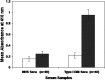
Results: DNA glycation with MG, Lys and Cu2+ results in the formation of CEdG (marker of DNA glycation) which was confirmed by LC-MS. The intermediate stages of glycation were confirmed by ESI-MS technique. Serum from diabetes patients exhibited enhanced binding and specificity for glycated DNA as compared to native form.
Conclusions: Glycation of DNA has resulted in structural perturbation causing generation of neo-antigenic epitopes thus recognizing auto-antibodies in diabetes.
Keywords: Auto-antibody; DNA; Diabetes mellitus; Glycation; MethylGlyoxal.
Figures


References
-
- Ahmed N. Advanced glycation endproducts-role in pathology of diabetic complications. Diabetes Res Clin Pract. 2006;67:3–21. - PubMed
-
- Mustafa I., Ahmad S., Dixit K., Moinuddin, Ahmad J., Ali A. Glycated human DNA is a preferred antigen for anti-DNA antibodies in diabetic patients. Diabetes Res Clin Pract. 2012;95:98–104. - PubMed
-
- Thornalley P.J. Cell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEs. Cell Mol Biol. 1998;44:1013–1023. - PubMed
-
- Stern D.M., Yan S.D., Yan S.F., Schmidt A.-M. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev. 2002;1:1–15. - PubMed
-
- McLellan A.C., Thornalley P.J., Benn J., Sonksen P.H. Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin Sci. 1994;87:21–29. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

