Examining the Link between Biofilm Formation and the Ability of Pathogenic Salmonella Strains to Colonize Multiple Host Species
- PMID: 29159172
- PMCID: PMC5581909
- DOI: 10.3389/fvets.2017.00138
Examining the Link between Biofilm Formation and the Ability of Pathogenic Salmonella Strains to Colonize Multiple Host Species
Abstract
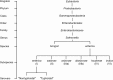
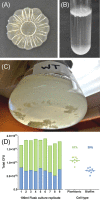
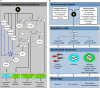
Salmonella are important pathogens worldwide and a predominant number of human infections are zoonotic in nature. The ability of strains to form biofilms, which is a multicellular behavior characterized by the aggregation of cells, is predicted to be a conserved strategy for increased persistence and survival. It may also contribute to the increasing number of infections caused by ingestion of contaminated fruits and vegetables. There is a correlation between biofilm formation and the ability of strains to colonize and replicate within the intestines of multiple host species. These strains predominantly cause localized gastroenteritis infections in humans. In contrast, there are salmonellae that cause systemic, disseminated infections in a select few host species; these "invasive" strains have a narrowed host range, and most are unable to form biofilms. This includes host-restricted Salmonella serovar Typhi, which are only able to infect humans, and atypical gastroenteritis strains associated with the opportunistic infection of immunocompromised patients. From the perspective of transmission, biofilm formation is advantageous for ensuring pathogen survival in the environment. However, from an infection point of view, biofilm formation may be an anti-virulence trait. We do not know if the capacity to form biofilms prevents a strain from accessing the systemic compartments within the host or if loss of the biofilm phenotype reflects a change in a strain's interaction with the host. In this review, we examine the connections between biofilm formation, Salmonella disease states, degrees of host adaptation, and how this might relate to different transmission patterns. A better understanding of the dynamic lifecycle of Salmonella will allow us to reduce the burden of livestock and human infections caused by these important pathogens.
Keywords: Salmonella; biofilms; cellulose; curli; gastroenteritis; host adaptation.
Figures

References
-
- Nataro JP, Bopp CA, Fields PI, Kaper JB, Strockbine NA. Escherichia, Shigella, and Salmonella. 10th ed In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW, editors. Manual of Clinical Microbiology. Washington, DC: American Society of Microbiology; (2011). p. 603–26.
-
- Le Minor L, Popoff MY. Designation of Salmonella enterica sp. nov., nom. rev., as the type and only species of the genus Salmonella: request for an opinion. Int J Syst Evol Microbiol (1987) 37:465–8.10.1099/00207713-37-4-465 - DOI
-
- Grimont P, Weill FX. Antigenic Formulae of the Salmonella Serovars. Paris: WHO Collaborating Centre for Reference and Research on Salmonella; (2007).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

