Immune response to vaccines is maintained in patients treated with dimethyl fumarate
- PMID: 29159204
- PMCID: PMC5688262
- DOI: 10.1212/NXI.0000000000000409
Immune response to vaccines is maintained in patients treated with dimethyl fumarate
Abstract
Objectives: To investigate the immune response to vaccinations in patients with relapsing forms of MS treated with delayed-release dimethyl fumarate (DMF) vs nonpegylated interferon (IFN).
Methods: In this open-label, multicenter study, patients received 3 vaccinations: (1) tetanus-diphtheria toxoid (Td) to test T-cell-dependent recall response, (2) pneumococcal vaccine polyvalent to test T-cell-independent humoral response, and (3) meningococcal (groups A, C, W-135, and Y) oligosaccharide CRM197 conjugate to test T-cell-dependent neoantigen response. Eligible patients were aged 18-55 years, diagnosed with relapsing-remitting MS (RRMS), and either treated for ≥6 months with an approved dose of DMF or for ≥3 months with an approved dose of nonpegylated IFN. Primary end point was the proportion of patients with ≥2-fold rise in antitetanus serum IgG levels from prevaccination to 4 weeks after vaccination.
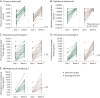
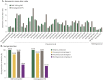
Results: Seventy-one patients (DMF treated, 38; IFN treated, 33) were enrolled. The mean age was 45.3 years (range 27-55); 86% were women. Responder rates (≥2-fold rise) to Td vaccination were comparable between DMF- and IFN-treated groups (68% vs 73%). Responder rates (≥2-fold rise) were also similar between DMF- and IFN-treated groups for diphtheria antitoxoid (58% vs 61%), pneumococcal serotype 3 (66% vs 79%), pneumococcal serotype 8 (95% vs 88%), and meningococcal serogroup C (53% vs 53%), all p > 0.05. In a post hoc analysis, no meaningful differences were observed between groups in the proportion of responders when stratified by age category or lymphocyte count.
Conclusions: DMF-treated patients mount an immune response to recall, neoantigens, and T-cell-independent antigens, which was comparable with that of IFN-treated patients and provided adequate seroprotection.
Clinicaltrialsgov identifier: NCT02097849.
Classification of evidence: This study provides Class II evidence that patients with RRMS treated with DMF respond to vaccinations comparably with IFN-treated patients.
Figures

References
-
- European Medicines Agency. Tecfidera gastro-resistant hard capsules [summary of product characteristics]. Available at: ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/hum.... Accessed August 11, 2016.
-
- Biogen, Inc Tecfidera [prescribing information]. Cambridge, MA: Biogen; 2017.
-
- Spencer CM, Crabtree-Hartman EC, Lehmann-Horn K, Cree BA, Zamvil SS. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm 2015;2:e76 doi: 10.1212/NXI.0000000000000076. - DOI - PMC - PubMed
-
- Fleischer V, Friedrich M, Rezk A, et al. . Treatment response to dimethyl fumarate is characterized by disproportionate CD8+ T cell reduction in MS. Mult Scler 2017:1352458517703799. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
