The Response of the Menstrual Cycle to Initiation of Hormonal Therapy in Transgender Men
- PMID: 29159311
- PMCID: PMC5685207
- DOI: 10.1089/trgh.2017.0023
The Response of the Menstrual Cycle to Initiation of Hormonal Therapy in Transgender Men
Abstract
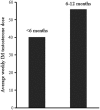
Purpose: Transition from female to male gender after initiation of hormonal therapy involves both phenotypic and physiological changes. The response to treatment can vary widely from person to person. In this study, we looked at the response of the menstrual cycle after the initiation of testosterone therapy and we wished to determine the time period and dose of testosterone leading to the cessation of menses in transgender men. Methods: A retrospective chart review of 114 transgender men (individuals assigned female gender at birth but later identify themselves as males) seen for hormonal therapy in our transgender clinic from 2006 through 2016. Results: Forty patients were excluded from the study as they were either not menstruating (19), started therapy elsewhere (13), had incomplete data (6), or were treated with transdermal testosterone (2). Of the 74 patients begun on low dose (20-40 mg weekly) intramuscular testosterone, 55% had cessation of menses within 6 months, 32% took more than 6 to 12 months, and 7% had not had complete cessation by 1 year and were prescribed progesterone (5% were given progesterone after menses had not ceased by 4 months for personal preference). The dose of testosterone correlated only loosely with the time of cessation (mean dose 40.1±8.1 mg/week for those <6 months and 56.1±12.0 mg/week for those who took 6-12 months). Conclusion: The initiation of low to moderate testosterone was sufficient in leading to menstrual cessation in the majority of patients by 6 months and nearly all by 1 year. There was individual variability in the time to cessation with little correlation to testosterone dose.
Keywords: female to male transgender; menstrual cycles; testosterone.
Conflict of interest statement
No competing financial interests exist.
Figures


Similar articles
-
Menstrual Suppression in Adolescent and Young Adult Transgender Males.J Pediatr Adolesc Gynecol. 2023 Apr;36(2):116-121. doi: 10.1016/j.jpag.2022.10.007. Epub 2022 Oct 27. J Pediatr Adolesc Gynecol. 2023. PMID: 37938039
-
Physical and Psychological Effects of Gender-Affirming Hormonal Treatment Using Intramuscular Testosterone Enanthate in Japanese Transgender Men.Sex Med. 2021 Apr;9(2):100306. doi: 10.1016/j.esxm.2020.100306. Epub 2021 Feb 1. Sex Med. 2021. PMID: 33540366 Free PMC article.
-
Subcutaneous Testosterone: An Effective Delivery Mechanism for Masculinizing Young Transgender Men.LGBT Health. 2014 Sep;1(3):165-7. doi: 10.1089/lgbt.2014.0018. Epub 2014 Jun 26. LGBT Health. 2014. PMID: 26789709
-
Hormonal Contraceptives, Intrauterine Devices, Gonadotropin-releasing Hormone Analogues and Testosterone: Menstrual Suppression in Special Adolescent Populations.J Pediatr Adolesc Gynecol. 2019 Sep;32(5S):S23-S29. doi: 10.1016/j.jpag.2019.04.007. Epub 2019 Apr 11. J Pediatr Adolesc Gynecol. 2019. PMID: 30980941 Review.
-
Sleep regulation and sex hormones exposure in men and women across adulthood.Pathol Biol (Paris). 2014 Oct;62(5):302-10. doi: 10.1016/j.patbio.2014.07.005. Epub 2014 Sep 11. Pathol Biol (Paris). 2014. PMID: 25218407 Review.
Cited by
-
One-third of amenorrheic transmasculine people on testosterone ovulate.Cell Rep Med. 2024 Mar 19;5(3):101440. doi: 10.1016/j.xcrm.2024.101440. Epub 2024 Feb 22. Cell Rep Med. 2024. PMID: 38402622 Free PMC article.
-
Persistent vaginal bleeding during gender-affirming hormone therapy in transgender men.J Endocrinol Invest. 2024 Aug;47(8):2053-2060. doi: 10.1007/s40618-023-02296-w. Epub 2024 Feb 1. J Endocrinol Invest. 2024. PMID: 38300501
-
Effects of acute aerobic exercise on arterial stiffness in transgender men.Front Physiol. 2023 Oct 31;14:1294284. doi: 10.3389/fphys.2023.1294284. eCollection 2023. Front Physiol. 2023. PMID: 38028805 Free PMC article.
-
"Just because I don't bleed, doesn't mean I don't go through it": Expanding knowledge on trans and non-binary menstruators.Int J Transgend Health. 2021 Sep 25;22(1-2):113-125. doi: 10.1080/15532739.2020.1819507. eCollection 2021. Int J Transgend Health. 2021. PMID: 34568874 Free PMC article.
-
REPRODUCTIVE HEALTH IN TRANS AND GENDER DIVERSE PATIENTS: Contraception considerations in transmasculine and gender diverse adolescents and young adults.Reproduction. 2025 Jan 2;169(1):e240080. doi: 10.1530/REP-24-0080. Print 2025 Jan 1. Reproduction. 2025. PMID: 39418097 Free PMC article. Review.
References
-
- Shields JP, Cohen R, Glassman JR, et al. . Estimating population size and demographic characteristics of lesbian, gay, bisexual, and transgender youth in middle school. J Adolesc Health. 2013;17:248–250 - PubMed
-
- Leinung MC, Urizar MF, Patel N, et al. . Endocrine treatment of transsexual persons: extensive personal experience. Endocr Pract. 2013;19:644–650 - PubMed
-
- Ahmad S, Joseph J, Leinung MC. A cross-sectional study of clinical and psychosocial characteristics of a cohort of 371 transgender patient treated at Albany Medical Center. Endocrine Rev. 2016;37(2:Supplement): abstract FRI-139.
-
- Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. . Endocrine treatment of transsexual persons: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94:3132–3154 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
