Acute intermittent hypoxia with concurrent hypercapnia evokes P2X and TRPV1 receptor-dependent sensory long-term facilitation in naïve carotid bodies
- PMID: 29159869
- PMCID: PMC6068228
- DOI: 10.1113/JP275001
Acute intermittent hypoxia with concurrent hypercapnia evokes P2X and TRPV1 receptor-dependent sensory long-term facilitation in naïve carotid bodies
Abstract
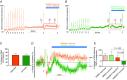
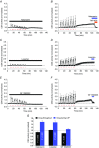
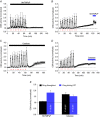
Key points: Activity-dependent plasticity can be induced in carotid body (CB) chemosensory afferents without chronic intermittent hypoxia (CIH) preconditioning by acute intermittent hypoxia coincident with bouts of hypercapnia (AIH-Hc). Several properties of this acute plasticity are shared with CIH-dependent sensory long-term facilitation (LTF) in that induction is dependent on 5-HT, angiotensin II, protein kinase C and reactive oxygen species. Several properties differ from CIH-dependent sensory LTF; H2 O2 appears to play no part in induction, whereas maintenance requires purinergic P2X2/3 receptor activation and is dependent on transient receptor potential vanilloid type 1 (TRPV1) receptor sensitization. Because P2X2/3 and TRPV1 receptors are located in carotid sinus nerve (CSN) terminals but not presynaptic glomus cells, a primary site of the acute AIH-Hc induced sensory LTF appears to be postsynaptic. Our results obtained in vivo suggest a role for TRPV1-dependent CB activity in acute sympathetic LTF. We propose that P2X-TRPV1-receptor-dependent sensory LTF may constitute an important early mechanism linking sleep apnoea with hypertension and/or cardiovascular disease.
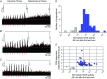
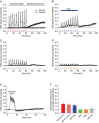
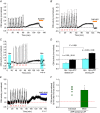
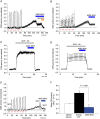
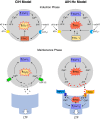
Abstract: Apnoeas constitute an acute existential threat to neonates and adults. In large part, this threat is detected by the carotid bodies, which are the primary peripheral chemoreceptors, and is combatted by arousal and acute cardiorespiratory responses, including increased sympathetic output. Similar responses occur with repeated apnoeas but they continue beyond the last apnoea and can persist for hours [i.e. ventilatory and sympathetic long-term facilitation (LTF)]. These long-term effects may be adaptive during acute episodic apnoea, although they may prolong hypertension causing chronic cardiovascular impairment. We report a novel mechanism of acute carotid body (CB) plasticity (sensory LTF) induced by repeated apnoea-like stimuli [i.e. acute intermittent hypoxia coincident with bouts of hypercapnia (AIH-Hc)]. This plasticity did not require chronic intermittent hypoxia preconditioning, was dependent on P2X receptors and protein kinase C, and involved heat-sensitive transient receptor potential vanilloid type 1 (TRPV1) receptors. Reactive oxygen species (O2 ·¯) were involved in initiating plasticity only; no evidence was found for H2 O2 involvement. Angiotensin II and 5-HT receptor antagonists, losartan and ketanserin, severely reduced CB responses to individual hypoxic-hypercapnic challenges and prevented the induction of sensory LTF but, if applied after AIH-Hc, failed to reduce plasticity-associated activity. Conversely, TRPV1 receptor antagonism had no effect on responses to individual hypoxic-hypercapnic challenges but reduced plasticity-associated activity by ∼50%. Further, TRPV1 receptor antagonism in vivo reduced sympathetic LTF caused by AIH-Hc, although only if the CBs were functional. These data demonstrate a new mechanism of CB plasticity and suggest P2X-TRPV1-dependent sensory LTF as a novel target for pharmacological intervention in some forms of neurogenic hypertension associated with recurrent apnoeas.
Keywords: carotid body; hypoxia-hypercapnia; sensory long term facilitation.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures

Comment in
-
Purinergic signalling and TRPV1 receptors are associated with the carotid body plasticity induced by an apnoea-like stimulus.J Physiol. 2018 Aug;596(15):2961-2962. doi: 10.1113/JP275889. Epub 2018 Mar 26. J Physiol. 2018. PMID: 29478282 Free PMC article. No abstract available.
References
-
- Bao G, Randhawa PM & Fletcher EC (1997). Acute blood pressure elevation during repetitive hypocapnic and eucapnic hypoxia in rats. J Appl Physiol 82, 1071–1078. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD & Julius D (1997). The capsaicin receptor: a heat‐activated ion channel in the pain pathway. Nature 389, 816–824. - PubMed
-
- Chin K, Hiras M, Kuriyama T, Fukui M, Kuno K, Sagawa Y & Ohi M (1997). Changes in the arterial PCO2 during a single night's sleep in patirnts with obstructive sleep apnea. Intern Med 36, 454–460. - PubMed
-
- Cummings KJ & Wilson RJA (2005). Time‐dependent modulation of carotid body afferent activity during and after intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 288, R1571–R1580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

