Predictive hyperglycemia and hypoglycemia minimization: In-home double-blind randomized controlled evaluation in children and young adolescents
- PMID: 29159870
- PMCID: PMC5951790
- DOI: 10.1111/pedi.12603
Predictive hyperglycemia and hypoglycemia minimization: In-home double-blind randomized controlled evaluation in children and young adolescents
Abstract
Objective: The primary objective of this trial was to evaluate the feasibility, safety, and efficacy of a predictive hyperglycemia and hypoglycemia minimization (PHHM) system vs predictive low glucose suspension (PLGS) alone in optimizing overnight glucose control in children 6 to 14 years old.
Research design and methods: Twenty-eight participants 6 to 14 years old with T1D duration ≥1 year with daily insulin therapy ≥12 months and on insulin pump therapy for ≥6 months were randomized per night into PHHM mode or PLGS-only mode for 42 nights. The primary outcome was percentage of time in sensor-measured range 70 to 180 mg/dL in the overnight period.
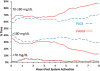
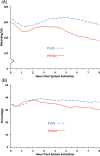
Results: The addition of automated insulin delivery with PHHM increased time in target range (70-180 mg/dL) from 66 ± 11% during PLGS nights to 76 ± 9% during PHHM nights (P<.001), without increasing hypoglycemia as measured by time below various thresholds. Average morning blood glucose improved from 176 ± 28 mg/dL following PLGS nights to 154 ± 19 mg/dL following PHHM nights (P<.001).
Conclusions: The PHHM system was effective in optimizing overnight glycemic control, significantly increasing time in range, lowering mean glucose, and decreasing glycemic variability compared to PLGS alone in children 6 to 14 years old.
Keywords: automated insulin delivery; continuous glucose monitoring; type 1 diabetes.
© 2017 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
References
-
- Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Hypoglycemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care. 1997;20(1):22–25. - PubMed
-
- The Diabetes Control and Complications Trial Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46(2):271–286. - PubMed
-
- Jones TW, Porter P, Sherwin RS, et al. Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med. 1998;338(23):1657–1662. - PubMed
-
- Ly TT, Jones TW, Griffiths A, et al. Hypoglycemia does not change the threshold for arousal from sleep in adolescents with type 1 diabetes. Diabetes Technol Ther. 2012;14(2):101–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical