Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance
- PMID: 29160310
- PMCID: PMC5754234
- DOI: 10.1038/nature25015
Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance
Erratum in
-
Author Correction: Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance.Nature. 2019 Jul;571(7766):E10. doi: 10.1038/s41586-019-1351-8. Nature. 2019. PMID: 31270456
Abstract
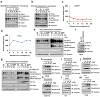
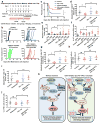
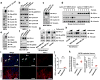
Treatments that target immune checkpoints, such as the one mediated by programmed cell death protein 1 (PD-1) and its ligand PD-L1, have been approved for treating human cancers with durable clinical benefit. However, many patients with cancer fail to respond to compounds that target the PD-1 and PD-L1 interaction, and the underlying mechanism(s) is not well understood. Recent studies revealed that response to PD-1-PD-L1 blockade might correlate with PD-L1 expression levels in tumour cells. Hence, it is important to understand the mechanistic pathways that control PD-L1 protein expression and stability, which can offer a molecular basis to improve the clinical response rate and efficacy of PD-1-PD-L1 blockade in patients with cancer. Here we show that PD-L1 protein abundance is regulated by cyclin D-CDK4 and the cullin 3-SPOP E3 ligase via proteasome-mediated degradation. Inhibition of CDK4 and CDK6 (hereafter CDK4/6) in vivo increases PD-L1 protein levels by impeding cyclin D-CDK4-mediated phosphorylation of speckle-type POZ protein (SPOP) and thereby promoting SPOP degradation by the anaphase-promoting complex activator FZR1. Loss-of-function mutations in SPOP compromise ubiquitination-mediated PD-L1 degradation, leading to increased PD-L1 levels and reduced numbers of tumour-infiltrating lymphocytes in mouse tumours and in primary human prostate cancer specimens. Notably, combining CDK4/6 inhibitor treatment with anti-PD-1 immunotherapy enhances tumour regression and markedly improves overall survival rates in mouse tumour models. Our study uncovers a novel molecular mechanism for regulating PD-L1 protein stability by a cell cycle kinase and reveals the potential for using combination treatment with CDK4/6 inhibitors and PD-1-PD-L1 immune checkpoint blockade to enhance therapeutic efficacy for human cancers.
Conflict of interest statement
GF has patents/pending royalties on the PD-1 pathway from Roche, Merck, Bristol-Myers-Squibb, EMD-Serono, Boehringer-Ingelheim, AstraZeneca, Dako and Novartis. GF has served on advisory boards for CoStim, Novartis, Roche, Eli Lilly, Bristol-Myers-Squibb, Seattle Genetics, Bethyl Laboratories, Xios, and Quiet. PS in a consultant and a recipient of a research grant from Novartis. No potential conflicts of interests were disclosed by other authors.
The authors declare no competing financial interests.
Figures











References
-
- Gotwals P, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17:286–301. - PubMed
-
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. - PubMed
-
- Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14:561–584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA083688/CA/NCI NIH HHS/United States
- R01 CA200651/CA/NCI NIH HHS/United States
- P01 CA080111/CA/NCI NIH HHS/United States
- P50 CA101942/CA/NCI NIH HHS/United States
- R01 GM094777/GM/NIGMS NIH HHS/United States
- R01 CA200573/CA/NCI NIH HHS/United States
- R01 CA202634/CA/NCI NIH HHS/United States
- K99 CA212292/CA/NCI NIH HHS/United States
- R01 CA177910/CA/NCI NIH HHS/United States
- R01 GM089763/GM/NIGMS NIH HHS/United States
- R01 CA132740/CA/NCI NIH HHS/United States
- R01 CA229307/CA/NCI NIH HHS/United States
- R01 CA190509/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

