Organocatalytic Access to Enantioenriched Spirooxindole-Based 4-Methyleneazetidines
- PMID: 29160827
- PMCID: PMC6150293
- DOI: 10.3390/molecules22112016
Organocatalytic Access to Enantioenriched Spirooxindole-Based 4-Methyleneazetidines
Abstract
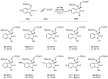
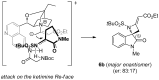
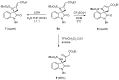
This work describes the synthesis of enantioenriched spiro compounds, incorporating the azetidine and the oxindole motifs. The preparation relies on a formal [2 + 2] annulation reaction of isatin-derived N-tert-butylsulfonyl ketimines with allenoates. The asymmetric induction is secured by an organocatalytic strategy, exploiting a bifunctional cinchona-type β-isocupridine-based catalyst. Some post-transformation products, including unexpected spiropyrroline and 3,3-disubstituted oxindole derivatives, are also presented.
Keywords: azetidines; cinchona-based catalysts; organocatalysis; spirooxindoles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

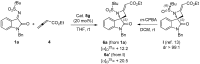
References
-
- Liu J., Sun Y., Zhang X., Liang X., Wu Y., Wang Y., Jiang X. Spirooxindoles, a potential novel class of anti-inflammatory agents. Inflamm. Cell Signal. 2014;1:e372. doi: 10.14800/ics.372. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

