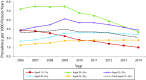
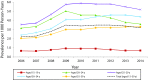
Declines in Anogenital Warts Among Age Groups Most Likely to Be Impacted by Human Papillomavirus Vaccination, United States, 2006-2014
- PMID: 29161070
- PMCID: PMC5719685
- DOI: 10.2105/AJPH.2017.304119
Declines in Anogenital Warts Among Age Groups Most Likely to Be Impacted by Human Papillomavirus Vaccination, United States, 2006-2014
Abstract
Objectives: To detect decreases in anogenital warts (AGW) among sex and age groups likely to be affected by human papillomavirus vaccination.
Methods: We estimated annual AGW prevalence during 2006 to 2014 using health care claims among US private health insurance enrollees aged 15 to 39 years. We derived AGW diagnoses using 1 of the following: (1) condylomata acuminata diagnosis, (2) viral wart diagnosis combined with a benign anogenital neoplasm diagnosis or destruction or excision of an anogenital lesion, or (3) AGW medication combined with a benign anogenital neoplasm diagnosis or destruction or excision of an anogenital lesion.
Results: Prevalence decreased during 2008 to 2014 among females aged 15 to 19 years (annual percentage change [APC] = -14.1%; P < .001) and during 2009 to 2014 among women aged 20 to 24 years (APC = -12.9%; P < .001) and among women aged 25 to 29 years (APC = -6.0%; P = .001). We observed significant declines among men aged 20 to 24 years (APC = -6.5%; P = .005). Prevalence increased or was stable in all other sex and age groups.
Conclusions: We observed AGW decreases among females in the age groups most likely to be affected by human papillomavirus vaccination and decreases in men aged 20 to 24 years. Decreased prevalence in young men is likely attributable to herd protection from vaccination among females.
Figures
Comment in
-
HPV Vaccination: Increase Uptake Now to Reduce Cancer.Am J Public Health. 2018 Jan;108(1):23-24. doi: 10.2105/AJPH.2017.304184. Am J Public Health. 2018. PMID: 29211517 Free PMC article. No abstract available.
References
-
- Garland SM, Steben M, Sings HL et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199(6):805–814. - PubMed
-
- Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR-2):1–24. - PubMed
-
- Centers for Disease Control and Prevention. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59(20):630–632. - PubMed
-
- Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705–1708. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical