A New Frontier in Immunometabolism. Cholesterol in Lung Health and Disease
- PMID: 29161079
- PMCID: PMC5711269
- DOI: 10.1513/AnnalsATS.201702-136AW
A New Frontier in Immunometabolism. Cholesterol in Lung Health and Disease
Abstract
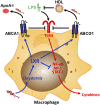
The lung has a unique relationship to cholesterol that is shaped by its singular physiology. On the one hand, the lungs receive the full cardiac output and have a predominant dependence on plasma lipoprotein uptake for their cholesterol supply. On the other hand, surfactant lipids, including cholesterol, are continually susceptible to oxidation owing to direct environmental exposure and must be cleared or recycled because of the very narrow biophysical mandates placed upon surfactant lipid composition. Interestingly, increased lipid-laden macrophage "foam cells" have been noted in a wide range of human lung pathologies. This suggests that lipid dysregulation may be a unifying and perhaps contributory event in chronic lung disease pathogenesis. Recent studies have shown that perturbations in intracellular cholesterol trafficking critically modify the immune response of macrophages and other cells. This minireview discusses literature that has begun to demonstrate the importance of regulated cholesterol traffic through the lung to pulmonary immunity, inflammation, and fibrosis. This emerging recognition of coupling between immunity and lipid homeostasis in the lung presents potentially transformative concepts for understanding lung disease and may also offer novel and exciting avenues for therapeutic development.
Keywords: innate immunity; lipoproteins; liver X receptors; oxysterols.
Figures

References
-
- Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA, Tabas I. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-κB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280:21763–21772. - PubMed
-
- Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via Toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

