Cadherin-1 and cadherin-3 cooperation determines the aggressiveness of pancreatic ductal adenocarcinoma
- PMID: 29161242
- PMCID: PMC5830586
- DOI: 10.1038/bjc.2017.411
Cadherin-1 and cadherin-3 cooperation determines the aggressiveness of pancreatic ductal adenocarcinoma
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is characterised by an extensive tissue invasion and an early formation of metastasis. Alterations in the expression of cadherins have been reported in PDAC. Yet, how these changes contribute to tumour progression is poorly understood. Here, we investigated the relationship between cadherins expression and PDAC development.
Methods: Cadherins expression was assessed by immunostaining in both human and murine tissue specimens. We have generated pancreatic cancer cell lines expressing both cadherin-1 and cadherin-3 or only one of these cadherins. Functional implications of such genetic alterations were analysed both in vitro and in vivo.
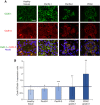
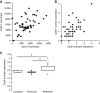
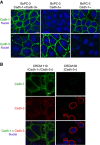
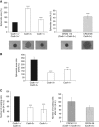
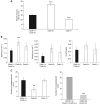
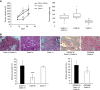
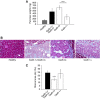
Results: Cadherin-3 is detected early at the plasma membrane during progression of pancreatic intraepithelial neoplasia 1 (PanIN-1) to PDAC. Despite tumoural cells turn on cadherin-3, a significant amount of cadherin-1 remains expressed at the cell surface during tumourigenesis. We found that cadherin-3 regulates tumour growth, while cadherin-1 drives type I collagen organisation in the tumour. In vitro assays showed that cadherins differentially participate to PDAC aggressiveness. Cadherin-3 regulates cell migration, whereas cadherin-1 takes part in the invadopodia activity.
Conclusions: Our results show differential, but complementary, roles for cadherins during PDAC carcinogenesis and illustrate how their expression conditions the PDAC aggressiveness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Al-Aynati MM, Radulovich N, Riddell RH, Tsao MS (2004) Epithelial-cadherin and beta-catenin expression changes in pancreatic intraepithelial neoplasia. Clin Cancer Res 10(4): 1235–1240. - PubMed
-
- Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grützmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Biankin AV, Grimmond SM Initiative APCG (2016) Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531(7592): 47–52. - PubMed
-
- Castro-Castro A, Marchesin V, Monteiro P, Lodillinsky C, Rossé C, Chavrier P (2016) Cellular and molecular mechanisms of MT1-MMP-dependent cancer cell invasion. Annu Rev Cell Dev Biol 32: 555–576. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

