The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
- PMID: 29161262
- PMCID: PMC5697827
- DOI: 10.1371/journal.pmed.1002446
The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
Abstract
Background: The specificity of nucleic acid amplification tests (NAATs) used for early infant diagnosis (EID) of HIV infection is <100%, leading some HIV-uninfected infants to be incorrectly identified as HIV-infected. The World Health Organization recommends that infants undergo a second NAAT to confirm any positive test result, but implementation is limited. Our objective was to determine the impact and cost-effectiveness of confirmatory HIV testing for EID programmes in South Africa.
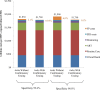
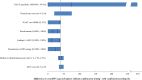
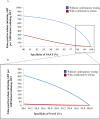
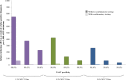
Method and findings: Using the Cost-effectiveness of Preventing AIDS Complications (CEPAC)-Pediatric model, we simulated EID testing at age 6 weeks for HIV-exposed infants without and with confirmatory testing. We assumed a NAAT cost of US$25, NAAT specificity of 99.6%, NAAT sensitivity of 100% for infants infected in pregnancy or at least 4 weeks prior to testing, and a mother-to-child transmission (MTCT) rate at 12 months of 4.9%; we simulated guideline-concordant rates of testing uptake, result return, and antiretroviral therapy (ART) initiation (100%). After diagnosis, infants were linked to and retained in care for 10 years (false-positive) or lifelong (true-positive). All parameters were varied widely in sensitivity analyses. Outcomes included number of infants with false-positive diagnoses linked to ART per 1,000 ART initiations, life expectancy (LE, in years) and per-person lifetime HIV-related healthcare costs. Both without and with confirmatory testing, LE was 26.2 years for HIV-infected infants and 61.4 years for all HIV-exposed infants; clinical outcomes for truly infected infants did not differ by strategy. Without confirmatory testing, 128/1,000 ART initiations were false-positive diagnoses; with confirmatory testing, 1/1,000 ART initiations were false-positive diagnoses. Because confirmatory testing averted costly HIV care and ART in truly HIV-uninfected infants, it was cost-saving: total cost US$1,790/infant tested, compared to US$1,830/infant tested without confirmatory testing. Confirmatory testing remained cost-saving unless NAAT cost exceeded US$400 or the HIV-uninfected status of infants incorrectly identified as infected was ascertained and ART stopped within 3 months of starting. Limitations include uncertainty in the data used in the model, which we examined with sensitivity and uncertainty analyses. We also excluded clinical harms to HIV-uninfected infants incorrectly treated with ART after false-positive diagnosis (e.g., medication toxicities); including these outcomes would further increase the value of confirmatory testing.
Conclusions: Without confirmatory testing, in settings with MTCT rates similar to that of South Africa, more than 10% of infants who initiate ART may reflect false-positive diagnoses. Confirmatory testing prevents inappropriate HIV diagnosis, is cost-saving, and should be adopted in all EID programmes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- World Health Organization. 2015 progress report on the global plan towards the elimination of new HIV infections among children and keeping their mothers alive. Geneva: World Health Organization; 2015. [cited 2017 Oct 20]. Available from: http://www.unaids.org/sites/default/files/media_asset/JC2774_2015Progres....
-
- Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364(9441):1236–43. doi: 10.1016/S0140-6736(04)17140-7 - DOI - PubMed
-
- Ghadrshenas A, Ben Amor Y, Chang J, Dale H, Sherman G, Vojnov L. Improved access to early infant diagnosis is a critical part of a child-centric prevention of mother-to-child transmission agenda. AIDS. 2013;27(Supp 2):S197–205. - PubMed
-
- Mayaux M-J, Burgard M, Teglas J, Cottalorda J, Krivine A, Puel J, et al. Neonatal characteristics in rapidly progressive perinatally acquired HIV-1 disease. JAMA. 1996;275(8):606–10. - PubMed
-
- Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–44. doi: 10.1056/NEJMoa0800971 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

