Switch to maraviroc with darunavir/r, both QD, in patients with suppressed HIV-1 was well tolerated but virologically inferior to standard antiretroviral therapy: 48-week results of a randomized trial
- PMID: 29161288
- PMCID: PMC5697828
- DOI: 10.1371/journal.pone.0187393
Switch to maraviroc with darunavir/r, both QD, in patients with suppressed HIV-1 was well tolerated but virologically inferior to standard antiretroviral therapy: 48-week results of a randomized trial
Abstract
Objectives: Primary study outcome was absence of treatment failure (virological failure, VF, or treatment interruption) per protocol at week 48.
Methods: Patients on 3-drug ART with stable HIV-1 RNA <50 copies/mL and CCR5-tropic virus were randomized 1:1 to maraviroc with darunavir/ritonavir qd (study arm) or continue current ART (continuation arm).
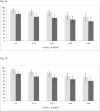
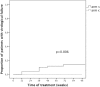
Results: In June 2015, 115 patients were evaluable for the primary outcome (56 study, 59 continuation arm). The study was discontinued due to excess of VF in the study arm (7 cases, 12.5%, vs 0 in the continuation arm, p = 0.005). The proportion free of treatment failure was 73.2% in the study and 59.3% in the continuation arm. Two participants in the study and 10 in the continuation arm discontinued therapy due to adverse events (p = 0.030). At VF, no emergent drug resistance was detected. Co-receptor tropism switched to non-R5 in one patient. Patients with VF reported lower adherence and had lower plasma drug levels. Femoral bone mineral density was significantly improved in the study arm.
Conclusion: Switching to maraviroc with darunavir/ritonavir qd in virologically suppressed patients was associated with improved tolerability but was virologically inferior to 3-drug therapy.
Conflict of interest statement
Figures

References
-
- Mugavero MJ, Napravnik S, Cole SR, Eron JJ, Lau B, Crane HM, et al. Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) Cohort Study. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis. 2011. November;53(9):927–35. doi: 10.1093/cid/cir526 - DOI - PMC - PubMed
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; January 28, 2016. (17 Jun 2017, date last accessed).
-
- European AIDS Clinical Society (EACS). Guidelines: Clinical Management and Treatment of HIV Infected Adults in Europe, Prevention and Management of Non-Infectious Comorbidities in HIV, Version 8.2, January 2017. http://www.europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/EACSG... (17 Jun 2017, date last accessed).
-
- Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults 2016. Recommendations of the International Antiviral Society-USA Panel. JAMA. 2016; 316(2):191–210. doi: 10.1001/jama.2016.8900 - DOI - PMC - PubMed
-
- Grant PM, Cotter AG. Tenofovir and bone health. Curr Opin HIV AIDS. 2016. May;11(3):326–32. doi: 10.1097/COH.0000000000000248 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

