In-vivo effects of intraocular and intracranial pressures on the lamina cribrosa microstructure
- PMID: 29161320
- PMCID: PMC5697865
- DOI: 10.1371/journal.pone.0188302
In-vivo effects of intraocular and intracranial pressures on the lamina cribrosa microstructure
Abstract
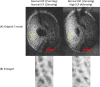
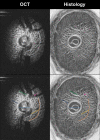
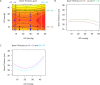
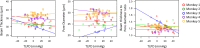
There is increasing clinical evidence that the eye is not only affected by intraocular pressure (IOP), but also by intracranial pressure (ICP). Both pressures meet at the optic nerve head of the eye, specifically the lamina cribrosa (LC). The LC is a collagenous meshwork through which all retinal ganglion cell axons pass on their way to the brain. Distortion of the LC causes a biological cascade leading to neuropathy and impaired vision in situations such as glaucoma and idiopathic intracranial hypertension. While the effect of IOP on the LC has been studied extensively, the coupled effects of IOP and ICP on the LC remain poorly understood. We investigated in-vivo the effects of IOP and ICP, controlled via cannulation of the eye and lateral ventricle in the brain, on the LC microstructure of anesthetized rhesus monkeys eyes using the Bioptigen spectral-domain optical coherence tomography (OCT) device (Research Triangle, NC). The animals were imaged with their head upright and the rest of their body lying prone on a surgical table. The LC was imaged at a variety of IOP/ICP combinations, and microstructural parameters, such as the thickness of the LC collagenous beams and diameter of the pores were analyzed. LC microstructure was confirmed by histology. We determined that LC microstructure deformed in response to both IOP and ICP changes, with significant interaction between the two. These findings emphasize the importance of considering both IOP and ICP when assessing optic nerve health.
Conflict of interest statement
Figures




References
-
- Quigley HA. Glaucoma. The Lancet. 2011;377(9774):1367–77. - PubMed
-
- Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 2000;19(3):297–321. - PubMed
-
- Burgoyne CF. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp Eye Res. 2011. August;93(2):120–32. doi: 10.1016/j.exer.2010.09.005 - DOI - PMC - PubMed
-
- Kwon YH, Fingert JH, Kuehn MH, Alward WLM. Primary open-angle glaucoma. N Engl J Med. 2009. March 12;360(11):1113–24. doi: 10.1056/NEJMra0804630 - DOI - PMC - PubMed
-
- Tso M, Hayreh S. Optic disc edema in raised intracranial pressure: IV. Axoplasmic transport in experimental papilledema. Arch Ophthalmol. 1977. August 1;95(8):1458–62. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

