Astrocyte-Specific Deletion of Sox2 Promotes Functional Recovery After Traumatic Brain Injury
- PMID: 29161339
- PMCID: PMC6659023
- DOI: 10.1093/cercor/bhx303
Astrocyte-Specific Deletion of Sox2 Promotes Functional Recovery After Traumatic Brain Injury
Abstract
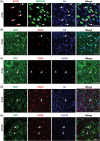
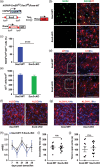
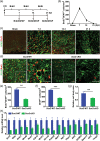
Injury to the adult brain induces activation of local astrocytes, which serves as a compensatory response that modulates tissue damage and recovery. However, the mechanism governing astrocyte activation during brain injury remains largely unknown. Here we provide in vivo evidence that SOX2, a transcription factor critical for stem cells and brain development, is also required for injury-induced activation of adult cortical astrocytes. Genome-wide chromatin immunoprecipitation-seq analysis of mouse cortical tissues reveals that SOX2 binds to regulatory regions of genes associated with signaling pathways that control glial cell activation, such as Nr2e1, Mmd2, Wnt7a, and Akt2. Astrocyte-specific deletion of Sox2 in adult mice greatly diminishes glial response to controlled cortical impact injury and, most unexpectedly, dampens injury-induced cortical loss and benefits behavioral recovery of mice after injury. Together, these results uncover an essential role of SOX2 in somatic cells under pathological conditions and indicate that SOX2-dependent astrocyte activation could be targeted for functional recovery after traumatic brain injury.
Figures


References
-
- Bani-Yaghoub M, Tremblay RG, Lei JX, Zhang D, Zurakowski B, Sandhu JK, Smith B, Ribecco-Lutkiewicz M, Kennedy J, Walker PR, et al. . 2006. Role of Sox2 in the development of the mouse neocortex. Dev Biol. 295:52–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

