Transfer and Metabolism of Cortisol by the Isolated Perfused Human Placenta
- PMID: 29161409
- PMCID: PMC5800837
- DOI: 10.1210/jc.2017-02140
Transfer and Metabolism of Cortisol by the Isolated Perfused Human Placenta
Abstract
Context: Fetal overexposure to glucocorticoids in utero is associated with fetal growth restriction and is postulated to be a key mechanism linking suboptimal fetal growth with cardiovascular disease in later life.
Objective: To develop a model to predict maternal-fetal glucocorticoid transfer. We hypothesized placental 11-β-hydroxysteroid dehydrogenase-type 2 (11β-HSD2) would be the major rate-limiting step in maternal cortisol transfer to the fetus.
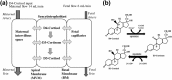
Design: We used a deuterated cortisol tracer in the ex vivo placental perfusion model, in combination with computational modeling, to investigate the role of interconversion of cortisol and its inactive metabolite cortisone on transfer of cortisol from mother to fetus.
Participants: Term placentas were collected from five women with uncomplicated pregnancies, at elective caesarean delivery.
Intervention: Maternal artery of the isolated perfused placenta was perfused with D4-cortisol.
Main outcome measures: D4-cortisol, D3-cortisone, and D3-cortisol were measured in maternal and fetal venous outflows.
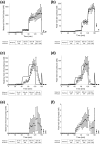
Results: D4-cortisol, D3-cortisone, and D3-cortisol were detected and increased in maternal and fetal veins as the concentration of D4-cortisol perfusion increased. D3-cortisone synthesis was inhibited when 11-β-hydroxysteroid dehydrogenase (11β-HSD) activity was inhibited. At the highest inlet concentration, only 3.0% of the maternal cortisol was transferred to the fetal circulation, whereas 26.5% was metabolized and 70.5% exited via the maternal vein. Inhibiting 11β-HSD activity increased the transfer to the fetus to 7.3% of the maternal input, whereas 92.7% exited via the maternal vein.
Conclusions: Our findings challenge the concept that maternal cortisol diffuses freely across the placenta and confirm that 11β-HSD2 acts as a major "barrier" to cortisol transfer to the fetus.
Copyright © 2017 Endocrine Society
Figures

References
-
- Stewart PM, Rogerson FM, Mason JI. Type 2 11 beta-hydroxysteroid dehydrogenase messenger ribonucleic acid and activity in human placenta and fetal membranes: its relationship to birth weight and putative role in fetal adrenal steroidogenesis. J Clin Endocrinol Metab. 1995;80(3):885–890. - PubMed
-
- Reynolds RM. Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis--2012 Curt Richter Award Winner. Psychoneuroendocrinology. 2013;38(1):1–11. - PubMed
-
- Jung C, Ho JT, Torpy DJ, Rogers A, Doogue M, Lewis JG, Czajko RJ, Inder WJ. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011;96(5):1533–1540. - PubMed
-
- Beitins IZ, Bayard F, Ances IG, Kowarski A, Migeon CJ. The metabolic clearance rate, blood production, interconversion and transplacental passage of cortisol and cortisone in pregnancy near term. Pediatr Res. 1973;7(5):509–519. - PubMed
-
- Brown RW, Chapman KE, Edwards CR, Seckl JR. Human placental 11 beta-hydroxysteroid dehydrogenase: evidence for and partial purification of a distinct NAD-dependent isoform. Endocrinology. 1993;132(6):2614–2621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

