Effects of Solvent and Temperature on Free Radical Formation in Electronic Cigarette Aerosols
- PMID: 29161504
- PMCID: PMC6471512
- DOI: 10.1021/acs.chemrestox.7b00116
Effects of Solvent and Temperature on Free Radical Formation in Electronic Cigarette Aerosols
Abstract
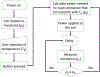
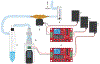
The ever-evolving market of electronic cigarettes (e-cigarettes) presents a challenge for analyzing and characterizing the harmful products they can produce. Earlier we reported that e-cigarette aerosols can deliver high levels of reactive free radicals; however, there are few data characterizing the production of these potentially harmful oxidants. Thus, we have performed a detailed analysis of the different parameters affecting the production of free radical by e-cigarettes. Using a temperature-controlled e-cigarette device and a novel mechanism for reliably simulating e-cigarette usage conditions, including coil activation and puff flow, we analyzed the effects of temperature, wattage, and e-liquid solvent composition of propylene glycol (PG) and glycerol (GLY) on radical production. Free radicals in e-cigarette aerosols were spin-trapped and analyzed using electron paramagnetic resonance. Free radical production increased in a temperature-dependent manner, showing a nearly 2-fold increase between 100 and 300 °C under constant-temperature conditions. Free radical production under constant wattage showed an even greater increase when going from 10 to 50 W due, in part, to higher coil temperatures compared to constant-temperature conditions. The e-liquid PG content also heavily influenced free radical production, showing a nearly 3-fold increase upon comparison of ratios of 0:100 (PG:GLY) and 100:0 (PG:GLY). Increases in PG content were also associated with increases in aerosol-induced oxidation of biologically relevant lipids. These results demonstrate that the production of reactive free radicals in e-cigarette aerosols is highly solvent dependent and increases with an increase in temperature. Radical production was somewhat dependent on aerosol production at higher temperatures; however, disproportionately high levels of free radicals were observed at ≥100 °C despite limited aerosol production. Overall, these findings suggest that e-cigarettes can be designed to minimize exposure to these potentially harmful products.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures
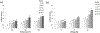
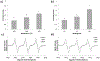
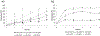
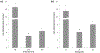
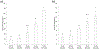
References
-
- Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. (2010) How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Centers for Disease Control and Prevention, Atlanta. - PubMed
-
- Bartalis J, Chan WG, and Wooten JB (2007) A new look at radicals in cigarette smoke. Anal. Chem 79, 5103–5106. - PubMed
-
- Dellinger B, Khachatryan L, Masko S, and Lomnicki S (2011) Free radicals in tobacco smoke. Mini-Rev. Org. Chem 8, 427–433.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

