Stereotactic body radiotherapy (SBRT) for locally advanced intrahepatic and extrahepatic cholangiocarcinoma
- PMID: 29162055
- PMCID: PMC5699184
- DOI: 10.1186/s12885-017-3788-1
Stereotactic body radiotherapy (SBRT) for locally advanced intrahepatic and extrahepatic cholangiocarcinoma
Abstract
Background: To evaluate the role of ablative radiotherapy doses in the treatment of hilar or intrahepatic cholangiocarcinoma (CCC) using stereotactic body radiotherapy (SBRT).
Methods: Consecutive patients treated from 2007 to 2016 with CCC were evaluated. Local control and toxicities were assessed every 3 months according to the Response Evaluation Criteria In Solid Tumors (RECIST) and the Common Terminology Criteria for Adverse Events v4.0, respectively. Overall survival (OS), local control (LC) and progression free survival were calculated from SBRT.
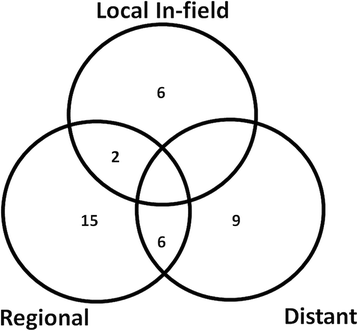
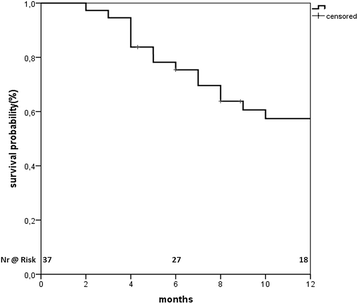
Results: Thirty seven patients with 43 lesions were retrospectively evaluated. The median dose delivered was 45 Gy (range 25-66 Gy) in 3-12 fractions, corresponding to a median equivalent dose in 2 Gy fractions (EQD210) of 56 (range 25-85) Gy. The median follow up was 24 months. The OS at 1 year was 56% with a median OS of 14 (95% CI: 7.8-20.2) months from start of SBRT and 22 (95% CI: 17.5-26.5) months from diagnosis. Eight lesions progressed locally. The local control rate (LC) at 1 year was 78%. The median progression free survival was 9 months (95% CI 2.8-15.2) 21 patients progressed in the liver but out of field and 15 progressed distantly. SBRT was well tolerated. Three patients (9%) developed a Grade III bleeding. Seven patients developed a cholangitis, one due to progression and the other because of a stent dysfunction 2-21(median 8) months from SBRT.
Conclusion: In patients with locally advanced cholangiocarcinoma, SBRT is a local treatment option with an acceptable toxicity profile which warrants further investigation in prospective trials.
Keywords: Intrahepatic and extrahepatic cholangiocarcinoma; SBRT; Stereotactic body radiotherapy.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the ethics committee of the University Hospital of Freiburg (No 519/16, date of approval: 27.10.2016) and was carried out in accordance with the Declaration of Helsinki, and the requirement for informed consent was waived because of the retrospective design.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical