Long-term prognosis of chronic cough: a prospective, observational cohort study
- PMID: 29162060
- PMCID: PMC5697342
- DOI: 10.1186/s12890-017-0496-1
Long-term prognosis of chronic cough: a prospective, observational cohort study
Abstract
Background: The long-term prognosis of chronic cough and its determinants need to be clarified.
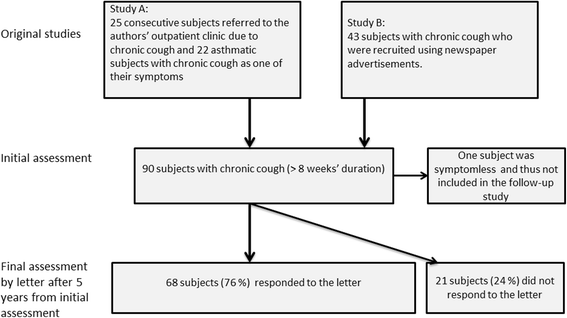
Methods: This is a prospective, observational cohort study. Eighty-nine unselected subjects with chronic (> 8 weeks' duration) cough were carefully investigated: Clinical examination, symptom questionnaire, Leicester Cough Questionnaire (LCQ), skin prick tests, ambulatory peak expiratory flow monitoring, spirometry before and after 0.4 mgs of salbutamol, exhaled nitric oxide concentration measurement, hypertonic saline cough provocation test, and histamine bronchial provocation test. After five years, a letter was sent to the subjects containing questions about continuation of cough, smoking, indoor exposures, presence of co-morbidities, and current medication. It also contained LCQ and Cough Clinic diagnostic questionnaire. Sixty-eight subjects (76%) responded.
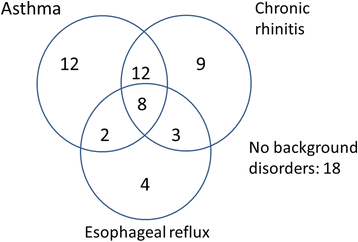
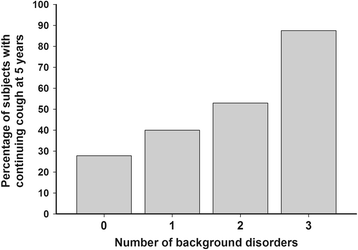
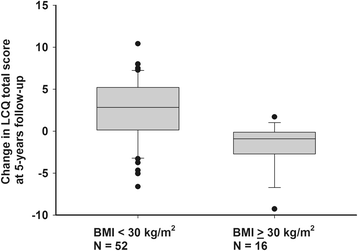
Results: At five years, continuing regular cough was present in 31 (46%) of the subjects and continuing impairment in cough-related quality of life (less than 1.3 points' improvement in LCQ) in 32 (47%). Continuing regular cough was associated with presence of chronic rhinitis or esophageal reflux disease, baseline mild airway responsiveness to histamine, and baseline strong cough responsiveness to hypertonic saline. Continuing impairment in cough-related quality of life was associated with high body mass index, absence of atopy, absence of pets, and high number of background disorders (esophageal reflux disease, asthma, or chronic rhinitis).
Conclusions: Almost half of subjects with chronic cough suffered of the disorder at five years from initial assessment. Several possible determinants of poor prognosis could be identified.
Keywords: Asthma; Chronic cough; Cough; Esophageal reflux disease; Rhinitis.
Conflict of interest statement
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. Research Ethic Committee, Hospital District of Northern Savo approved the protocol (40//2011). Written informed consent was obtained from all patients during the original studies [8, 9]. In the follow-up letter there was an information leaflet and the subject’s decision to reply was regarded as consent to participate the follow-up study.
Consent for publication
Not applicable.
Competing interests
HK has received payments for giving scientific lectures in gatherings organized by medical companies (Mundipharma Ltd., Orion Pharma Ltd., Oy, Eli Lilly Finland Ltd., Boehringer Ingelheim Finland Ltd) and has been sponsored by Takeda Leiras Ltd. and Mundipharma Ltd. to visit international scientific meetings. He owns shares of Orion Pharma Ltd. worth 25,000 euros.
AL has been sponsored by Takeda Leiras Ltd., Boehringer-Ingelheim Ltd. and Roche to visit international scientific meetings.
MP has got payments for giving scientific lectures in gatherings organized by medical companies (Boehringer-Ingelheim Finland Ltd., Roche, Takeda Leiras Ltd) and has been sponsored by Boehringer-Ingelheim Finland Ltd., Takeda Leiras Ltd. and Roche to visit international scientific meetings.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Centers for disease control and prevention, USA. National Ambulatory Medical Care Survey: 2010 Summary Tables. http://www.cdc.gov/nchs/data/ahcd/namcs_summary/2010_namcs_web_tables.pdf. Accessed 5 May 2017.
-
- Irwin RS, Baumann MH, Bolser DC, Boulet LP, Braman SS, Brightling CE, Brown KK, Canning BJ, Chang AB, Dicpinigaitis PV, Eccles R, Glomb WB, Goldstein LB, Graham LM, Hargreave FE, Kvale PA, Lewis SZ, McCool FD, McCrory DC, Prakash UB, Pratter MR, Rosen MJ, Schulman E, Shannon JJ, Smith Hammond C, Tarlo SM, American College of Chest Physicians (ACCP) Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:1S–23S. doi: 10.1378/chest.129.1_suppl.1S. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

