The effect of midazolam on pain control after knee arthroscopy: a systematic review and meta-analysis
- PMID: 29162135
- PMCID: PMC5697077
- DOI: 10.1186/s13018-017-0682-0
The effect of midazolam on pain control after knee arthroscopy: a systematic review and meta-analysis
Abstract
Background: Midazolam has some potential in pain control of patients undergoing knee arthroscopy. However, the results remain controversial. We conduct a systematic review and meta-analysis to explore the effect of midazolam on pain control after knee arthroscopy.
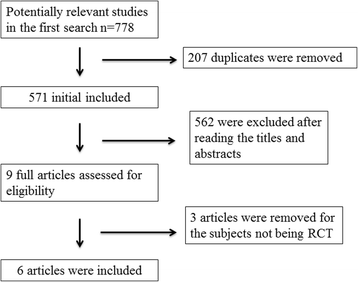
Methods: PubMed, EMbase, Web of science, EBSCO, and Cochrane library databases are systematically searched. Randomized controlled trials (RCTs) assessing the effect of midazolam on pain management after knee arthroscopy are included. Two investigators have independently searched articles, extracted the data, and assessed the quality of the included studies. This meta-analysis is performed using the random-effect model.
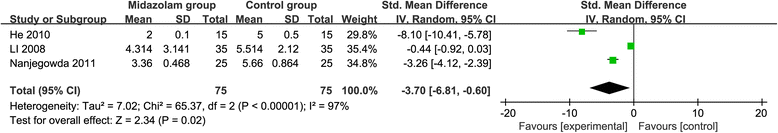
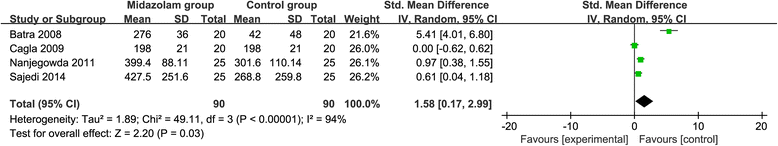
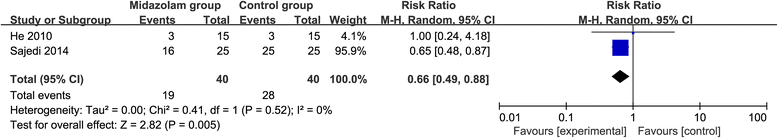
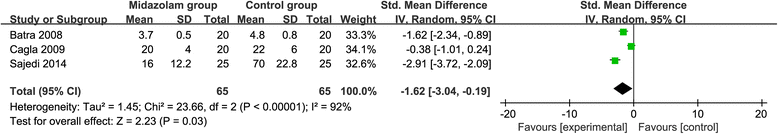
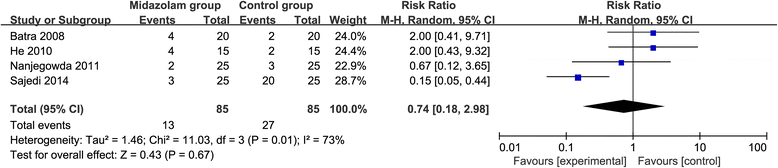
Results: Six RCTs are included in this meta-analysis. Compared with control intervention after knee arthroscopy, midazolam intervention can significantly reduce the pain scores (standard mean difference (Std. MD) = - 3.70; 95% confidence interval (CI) = - 6.81 to - 0.60; P = 0.02), the number of patients requiring analgesics (risk ratio (RR) = 0.66; 95% CI = 0.49 to 0.88; P = 0.005), and analgesic consumption (Std. MD = -1.62; 95% CI = - 3.04 to - 0.19; P = 0.03), as well as increase the time to first analgesic requirement (Std. MD = 1.58; 95% CI = 0.17 to 2.99; P = 0.03). In addition, midazolam intervention results in no increase in adverse events following knee arthroscopy (RR = 0.74; 95% CI = 0.18 to 2.98; P = 0.67).
Conclusions: Midazolam intervention is revealed to substantially reduce the pain scores, the number of patients requiring analgesics, and analgesic consumption, as well as improve the time to first analgesic requirement after knee arthroscopy.
Keywords: Knee arthroscopy; Meta-analysis; Midazolam; Pain control; Pain scores.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–157. doi: 10.1016/j.jpain.2015.12.008. - DOI - PubMed
-
- Gordon DB, de Leon-Casasola OA, Wu CL, Sluka KA, Brennan TJ, Chou R. Research gaps in practice guidelines for acute postoperative pain management in adults: findings from a review of the evidence for an American Pain Society Clinical Practice Guideline. J Pain. 2016;17:158–166. doi: 10.1016/j.jpain.2015.10.023. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

