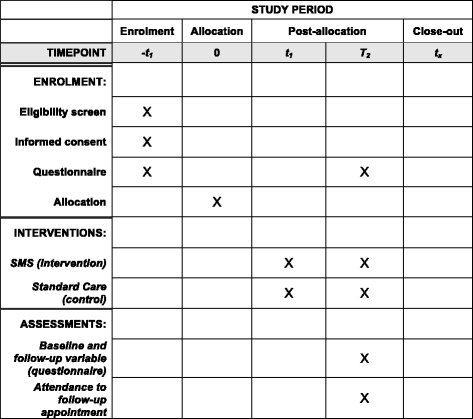
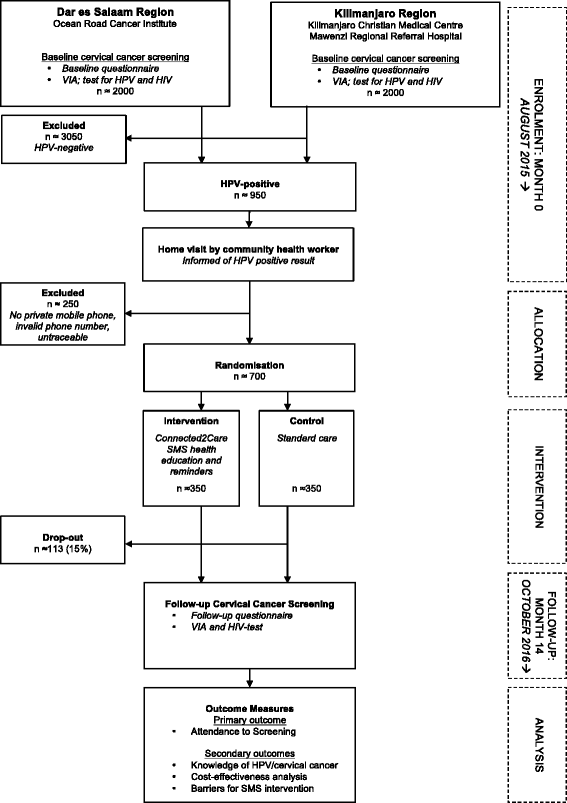
Text messages to increase attendance to follow-up cervical cancer screening appointments among HPV-positive Tanzanian women (Connected2Care): study protocol for a randomised controlled trial
- PMID: 29162148
- PMCID: PMC5698928
- DOI: 10.1186/s13063-017-2215-x
Text messages to increase attendance to follow-up cervical cancer screening appointments among HPV-positive Tanzanian women (Connected2Care): study protocol for a randomised controlled trial
Abstract
Background: Cervical cancer is a major health concern in Tanzania, caused by poor attendance for cervical cancer screening and follow-up of women at risk. Mobile telephone health interventions are proven effective tools to improve health behaviour in African countries. So far, no knowledge exists on how such interventions may perform in relation to cervical cancer screening in low-income settings. This study aims to assess the degree to which a Short Message Service (SMS) intervention can increase attendance at appointments among women who have tested positive for high-risk (HR) Human Papillomavirus (HPV) during cervical cancer screening.
Methods/design: Connected2Care is a non-blinded, multicentre, parallel-group, randomised controlled trial. Tanzanian women testing positive to HR HPV at inclusion are randomly assigned in an allocation ratio of 1:1 to the SMS intervention or the control group (standard care). In a period of 10 months, the intervention group will receive 15 one-directional health educative text messages and SMS reminders for their appointment. The total sample size will be 700 with 350 women in each study arm. Primary outcome is attendance rate for follow-up. Secondary objectives are cost-effectiveness, measured through incremental ratios, and knowledge of cervical cancer by a 16-item true/false scale questionnaire at baseline and follow-up. Barriers against implementing the intervention will be assessed in a mixed-methods sub-population study.
Discussion: This study may provide information on the potential effects, costs, and barriers in implementing an SMS intervention targeting a group of women who are followed up after testing positive for HR HPV and are, therefore, at increased risk of developing cervical cancer. This can guide decision-makers on the effective use of mobile technology in a low-income setting. Trial status: recruiting.
Trial registration: ClinicalTrials.gov, ID: NCT02509702 . Registered on 15 June 2015.
Keywords: Cervical cancer; HPV; Mobile phone; RCT; SMS intervention; Screening; Tanzania; mHealth.
Conflict of interest statement
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Effectiveness of One-Way Text Messaging on Attendance to Follow-Up Cervical Cancer Screening Among Human Papillomavirus-Positive Tanzanian Women (Connected2Care): Parallel-Group Randomized Controlled Trial.J Med Internet Res. 2020 Apr 2;22(4):e15863. doi: 10.2196/15863. J Med Internet Res. 2020. PMID: 32238335 Free PMC article. Clinical Trial.
-
Acceptability and feasibility of self-sampling and follow-up attendance after text message delivery of human papillomavirus results: A cross-sectional study nested in a cohort in rural Tanzania.Acta Obstet Gynecol Scand. 2021 Apr;100(4):802-810. doi: 10.1111/aogs.14117. Epub 2021 Mar 12. Acta Obstet Gynecol Scand. 2021. PMID: 33555038
-
Acceptability of text messages and knowledge change for cervical cancer screening: a Tanzanian mixed methods study.BMJ Open. 2022 Sep 19;12(9):e058450. doi: 10.1136/bmjopen-2021-058450. BMJ Open. 2022. PMID: 36123109 Free PMC article. Clinical Trial.
-
The Effectiveness of SMS Reminders on Appointment Attendance: a Meta-Analysis.J Med Syst. 2016 Apr;40(4):90. doi: 10.1007/s10916-016-0452-2. Epub 2016 Feb 6. J Med Syst. 2016. PMID: 26852337
-
Interventions using mHealth strategies to improve screening rates of cervical cancer: A scoping review.Prev Med. 2021 Feb;143:106387. doi: 10.1016/j.ypmed.2020.106387. Epub 2020 Dec 29. Prev Med. 2021. PMID: 33383069
Cited by
-
Introduction of HPV testing for cervical cancer screening in Central America: The Scale-Up project.Prev Med. 2020 Jun;135:106076. doi: 10.1016/j.ypmed.2020.106076. Epub 2020 Apr 2. Prev Med. 2020. PMID: 32247010 Free PMC article.
-
Targeted client communication via mobile devices for improving sexual and reproductive health.Cochrane Database Syst Rev. 2020 Jul 14;8(8):CD013680. doi: 10.1002/14651858.CD013680. Cochrane Database Syst Rev. 2020. PMID: 32779730 Free PMC article.
-
Outpatient Cancer Care Delivery in the Context of E-Oncology: A French Perspective on "Cancer outside the Hospital Walls".Cancers (Basel). 2019 Feb 14;11(2):219. doi: 10.3390/cancers11020219. Cancers (Basel). 2019. PMID: 30769858 Free PMC article.
-
One-way SMS and healthcare outcomes in Africa: Systematic review of randomised trials with meta-analysis.PLoS One. 2019 Jun 6;14(6):e0217485. doi: 10.1371/journal.pone.0217485. eCollection 2019. PLoS One. 2019. PMID: 31170176 Free PMC article.
-
Effectiveness of One-Way Text Messaging on Attendance to Follow-Up Cervical Cancer Screening Among Human Papillomavirus-Positive Tanzanian Women (Connected2Care): Parallel-Group Randomized Controlled Trial.J Med Internet Res. 2020 Apr 2;22(4):e15863. doi: 10.2196/15863. J Med Internet Res. 2020. PMID: 32238335 Free PMC article. Clinical Trial.
References
-
- Stewart W, Christopher PW. World Cancer Report 2014. 2014.
-
- Dartell M, Rasch V, Kahesa C, Mwaiselage J, Ngoma T, Junge J, et al. Human papillomavirus prevalence and type distribution in 3603 HIV-positive and HIV-negative women in the general population of Tanzania: the PROTECT study. Sex Transm Dis. 2012;39:201–8. doi: 10.1097/OLQ.0b013e31823b50ad. - DOI - PubMed
-
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs. Biological agents. Volume 100 B. A review of human carcinogens. Lyon: International Agency for Research on Cancer (IARC), WHO. http://monographs.iarc.fr/ENG/Monographs/vol100B/mono100B.pdf.
-
- International Agency for Cancer Research. Globocan 2012—Estimated cancer incidence, mortality and prevalence worldwide in 2012. Population Fact Sheets [Internet]. 2012 [cited 2015 Oct 28]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx#. cited 28 Oct 2015.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical