Surveillance and laboratory detection for non-polio enteroviruses in the European Union/European Economic Area, 2016
- PMID: 29162204
- PMCID: PMC5718392
- DOI: 10.2807/1560-7917.ES.2017.22.45.16-00807
Surveillance and laboratory detection for non-polio enteroviruses in the European Union/European Economic Area, 2016
Abstract
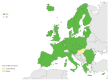
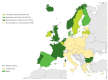
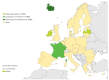
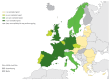
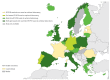
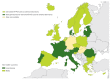
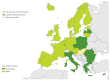
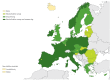
Enteroviruses (EVs) cause severe outbreaks of respiratory and neurological disease as illustrated by EV-D68 and EV-A71 outbreaks, respectively. We have mapped European laboratory capacity for identification and characterisation of non-polio EVs to improve preparedness to respond to (re)-emerging EVs linked to severe disease. An online questionnaire on non-polio EV surveillance and laboratory detection was submitted to all 30 European Union (EU)/European Economic Area (EEA) countries. Twenty-nine countries responded; 26 conducted laboratory-based non-polio EV surveillance, and 24 included neurological infections in their surveillance. Eleven countries have established specific surveillance for EV-D68 via sentinel influenza surveillance (n = 7), typing EV-positive respiratory samples (n = 10) and/or acute flaccid paralysis surveillance (n = 5). Of 26 countries performing non-polio EV characterisation/typing, 10 further characterised culture-positive EV isolates, whereas the remainder typed PCR-positive but culture-negative samples. Although 19 countries have introduced sequence-based EV typing, seven still rely entirely on virus isolation. Based on 2015 data, six countries typed over 300 specimens mostly by sequencing, whereas 11 countries characterised under 50 EV-positive samples. EV surveillance activity varied between EU/EEA countries, and did not always specifically target patients with neurological and/or respiratory infections. Introduction of sequence-based typing methods is needed throughout the EU/EEA to enhance laboratory capacity for the detection of EVs.
Keywords: Europe; laboratory methods; laboratory surveillance; molecular typing; respiratory infections; viral encephalitis; viral infections; viral meningitis.
Conflict of interest statement
Figures

References
-
- The Pirbright Institute. The Picornavirus Pages. Surrey: The Pirbright Institute; 7 Jun 2016. [Accessed 20 Jun 2016]. Available from: http://www.picornaviridae.com
-
- Knowles NJ, Hovi T, Hyypiä T, King AMQ, Lindberg AM, Pallansch MA, et al. Picornaviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Virus Taxonomy: Classification and Nomenclature of Viruses, Ninth Report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier; 2012 p. 855-880.
-
- Minor P, Brown F, Domingo E, Hoey E, King A, Knowles N, et al. Picornaviridae. In: Murphy FA, Fauquet CM, Bishop DHL, Ghabrial SA, Jarvis A, Martelli GP, et al., editors. Virus Taxonomy: Classification and Nomenclature of Viruses, Sixth report of the International Committee on Taxonomy of Viruses. Vienna: Springer-Verlag; 1995. p. 329-336.
-
- Grist NR, Bell EJ, Assaad F. Enteroviruses in human disease. Prog Med Virol. 1978;24:114-57. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
