Impact of rotavirus vaccine on acute gastroenteritis in children under 5 years in Senegal: Experience of sentinel site of the Albert Royer Children's Hospital in Dakar
- PMID: 29162319
- PMCID: PMC5959761
- DOI: 10.1016/j.vaccine.2017.10.061
Impact of rotavirus vaccine on acute gastroenteritis in children under 5 years in Senegal: Experience of sentinel site of the Albert Royer Children's Hospital in Dakar
Abstract
Background: Acute gastroenteritis (AGE) is a leading cause of morbidity and mortality among children <5 years of age in developing countries, with rotavirus being the most common infectious etiology. In November 2014, monovalent rotavirus vaccine was introduced in Senegal. We determined the impact of rotavirus vaccine on hospitalizations for all-cause and rotavirus related AGE in children <60 months of age.
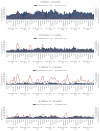
Methods: We examined two data sources from the national referral hospital. Using sentinel surveillance data from March 2011 to February 2017, we examined the proportion of AGE hospitalizations among children <60 months of age attributable to rotavirus, stratified by age groups (0-11, 12-23 and 24-59 months). Using pediatric logbook data from March 2010 to February 2017, we examined the proportion of all childhood hospitalizations attributable to AGE, among the same age groups.
Results: In sentinel surveillance, 673 patients <60 months were hospitalized for AGE, with 30% (203/673) due to rotavirus. In pre-vaccine years, the median proportion of rotavirus-positive hospitalizations was 42%; this proportion declined by 76% to 10% rotavirus positive in 2015-2016 (p < .001) and by 59% to 17% in 2016-2017 (p < .001). From the logbook data, among all children <60 months, a median of 11% of all hospitalizations in the pre-vaccine period were due to AGE, with 2015-2016 seeing a 16% decline (p < .001), to 9% of all hospitalizations, and 2016-2017 seeing a 39% decline (p < .001), to 7% of all hospitalizations. Declines in both rotavirus-associated and all-cause AGE hospitalizations were most marked among infants, with a suggestion of herd effect among older children seen in the surveillance data.
Conclusion: Rotavirus vaccine demonstrated a significant impact on rotavirus-associated hospitalizations and all-cause AGE hospitalizations in the first two seasons after vaccine introduction in Senegal. Our data support the continued use of this vaccine in national immunization program.
Keywords: Acute gastroenteritis; Monovalent rotavirus vaccine; Rotavirus; Senegal.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Groome MJ, Page N, Cortese MM, Moyes J, Zar HJ, Kapongo CN, et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case-control study. Lancet Infect Dis. 2014;14(11):1096–104. - PubMed
-
- Abeid KA, Jani B, Cortese MM, Kamugisha C, Mwenda JM, Pandu AS, et al. Monovalent rotavirus vaccine effectiveness and impact on rotavirus hospitalizations in Zanzibar, Tanzania: data from the first 3 years after introduction. J Infect Dis. 2016;215(2):183–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

