Effects of Intensive Blood Pressure Treatment on Acute Kidney Injury Events in the Systolic Blood Pressure Intervention Trial (SPRINT)
- PMID: 29162340
- PMCID: PMC5828778
- DOI: 10.1053/j.ajkd.2017.08.021
Effects of Intensive Blood Pressure Treatment on Acute Kidney Injury Events in the Systolic Blood Pressure Intervention Trial (SPRINT)
Abstract
Background: Treating to a lower blood pressure (BP) may increase acute kidney injury (AKI) events.
Study design: Data for AKI resulting in or during hospitalization or emergency department visits were collected as part of the serious adverse events reporting process of the Systolic Blood Pressure Intervention Trial (SPRINT).
Setting & participants: 9,361 participants 50 years or older with 1 or more risk factors for cardiovascular disease.
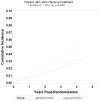
Interventions: Participants were randomly assigned to a systolic BP target of <120 (intensive arm) or <140mmHg (standard arm).
Outcomes & measurements: Primary outcome was the number of adjudicated AKI events. Secondary outcomes included severity of AKI and degree of recovery of kidney function after an AKI event. Baseline creatinine concentration was defined as the most recent SPRINT outpatient creatinine value before the date of the AKI event.
Results: There were 179 participants with AKI events in the intensive arm and 109 in the standard arm (3.8% vs 2.3%; HR, 1.64; 95% CI, 1.30-2.10; P<0.001). Of 288 participants with an AKI event, 248 (86.1%) had a single AKI event during the trial. Based on modified KDIGO (Kidney Disease: Improving Global Outcomes) criteria for severity of AKI, the number of AKI events in the intensive versus standard arm by KDIGO stage was 128 (58.5%) versus 81 (62.8%) for AKI stage 1, 42 (19.2%) versus 18 (14.0%) for AKI stage 2, and 42 (19.2%) versus 25 (19.4%) for AKI stage 3 (P=0.5). For participants with sufficient data, complete or partial resolution of AKI was seen for 169 (90.4%) and 9 (4.8%) of 187 AKI events in the intensive arm and 86 (86.9%) and 4 (4.0%) of 99 AKI events in the standard arm, respectively.
Limitations: Trial results are not generalizable to patients with diabetes mellitus or without risk factors for cardiovascular disease.
Conclusions: More intensive BP lowering resulted in more frequent episodes of AKI. Most cases were mild and most participants had complete recovery of kidney function.
Trial registration: Registered at ClinicalTrials.gov with study number NCT01206062.
Keywords: Acute kidney injury (AKI); BP lowering; adjudicated AKI episode; cardiovascular disease (CKD); chronic kidney disease (CKD); elderly; hypertension; kidney function; systolic blood pressure (SBP).
Copyright © 2017 National Kidney Foundation, Inc. All rights reserved.
Figures


References
-
- Behhdu S, et al. Effects of intensive systolic blood pressure lowering on incident chronic kidney disease and cardiovascular outcomes in non-chronic kidney disease subgroup in Systolic Blood Pressure Intervention Trial (SPRINT) J Am Soc Nephrol. 2016;27(Abstract Suppl):8A. - PubMed
-
- Rahmann M, et al. ASN oral presentation, Intensive blood pressure control and CKD progression in SPRINT. 2016 Nov 17;
-
- Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 TR000433/TR/NCATS NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- UL1 TR000075/TR/NCATS NIH HHS/United States
- P30 DK079626/DK/NIDDK NIH HHS/United States
- UL1 TR000093/TR/NCATS NIH HHS/United States
- UL1 TR000050/TR/NCATS NIH HHS/United States
- R01 DK091437/DK/NIDDK NIH HHS/United States
- HHSN268200900048C/HL/NHLBI NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- HHSN268200900040C/HL/NHLBI NIH HHS/United States
- HHSN268200900046C/HL/NHLBI NIH HHS/United States
- P30 GM103337/GM/NIGMS NIH HHS/United States
- UL1 TR001064/TR/NCATS NIH HHS/United States
- UL1 RR025752/RR/NCRR NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
- HHSN268200900049C/HL/NHLBI NIH HHS/United States
- HHSN268200900047C/HL/NHLBI NIH HHS/United States
- UL1 TR001427/TR/NCATS NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1 TR000073/TR/NCATS NIH HHS/United States
- UL1 RR025755/RR/NCRR NIH HHS/United States
- UL1 TR002003/TR/NCATS NIH HHS/United States
- UL1 TR000002/TR/NCATS NIH HHS/United States
- UL1 TR002240/TR/NCATS NIH HHS/United States
- UL1 TR000105/TR/NCATS NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- R21 DK106574/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

