Injectable Hydrogels for Localized Chemotherapy and Radiotherapy in Brain Tumors
- PMID: 29162424
- PMCID: PMC6093750
- DOI: 10.1016/j.xphs.2017.10.042
Injectable Hydrogels for Localized Chemotherapy and Radiotherapy in Brain Tumors
Abstract
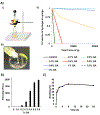
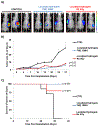
Overall survival of patients with newly diagnosed glioblastoma (GBM) remains dismal at 16 months with state-of-the-art treatment that includes surgical resection, radiation, and chemotherapy. GBM tumors are highly heterogeneous, and mechanisms for overcoming tumor resistance have not yet fully been elucidated. An injectable chitosan hydrogel capable of releasing chemotherapy (temozolomide [TMZ]) while retaining radioactive isotopes agents (iodine, [131I]) was used as a vehicle for localized radiation and chemotherapy, within the surgical cavity. Release from hydrogels loaded with TMZ or 131I was characterized in vitro and in vivo and their efficacy on tumor progression and survival on GBM tumors was also measured. The in vitro release of 131I was negligible over 42 days, whereas the TMZ was completely released over the first 48 h. 131I was completely retained in the tumor bed with negligible distribution in other tissues and that when delivered locally, the chemotherapy accumulated in the tumor at 10-fold higher concentrations than when delivered systemically. We found that the tumors were significantly decreased, and survival was improved in both treatment groups compared to the control group. Novel injectable chemo-radio-hydrogel implants may potentially improve the local control and overall outcome of aggressive, poor prognosis brain tumors.
Keywords: glioblastoma; hydrogels; injectable; localized chemotherapy; localized radiotherapy.
Copyright © 2018. Published by Elsevier Inc.
Conflict of interest statement
CONFLICT OF INTERESTS
Dr. Azab receives research support from Verastem, Selexys, Karyopharm, Cell Works, Cleave Bioscience, Glycomimetics, Abbvie and Vasculox; and is the founder and owner of Targeted Therapeutics LLC and Cellatrix LLC, however there has been no contribution of the aforementioned entities to the current study. Dr. de la Puente is a co-founder of Cellatrix LLC, however, there has been no contribution to the current study. Dr. de la Puente and Dr. Azab have a provisional patent application on the technology described in this manuscript. Other authors state no conflicts of interest.
Figures




References
-
- Kreth FW, Thon N, Siefert A, Tonn JC 2010. The place of interstitial brachytherapy and radiosurgery for low-grade gliomas. Advances and technical standards in neurosurgery 35:183–212. - PubMed
-
- de la Puente P, Azab AK 2014. Delivery systems for brachytherapy. J Control Release 192:19–28. - PubMed
-
- Chino K, Silvain D, Grace A, Stubbs J, Stea B 2008. Feasibility and safety of outpatient brachytherapy in 37 patients with brain tumors using the GliaSite Radiation Therapy System. Medical physics 35(7):3383–3388. - PubMed
-
- Mundinger F, Ostertag CB, Birg W, Weigel K 1980. Stereotactic treatment of brain lesions. Biopsy, interstitial radiotherapy (iridium-192 and iodine-125) and drainage procedures. Applied neurophysiology 43(3–5):198–204. - PubMed
-
- Mundinger F, Braus DF, Krauss JK, Birg W 1991. Long-term outcome of 89 low-grade brain-stem gliomas after interstitial radiation therapy. Journal of neurosurgery 75(5):740–746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

