PU.1-deficient mice are resistant to thioacetamide-induced hepatic fibrosis: PU.1 finely regulates Sirt1 expression via transcriptional promotion of miR-34a and miR-29c in hepatic stellate cells
- PMID: 29162670
- PMCID: PMC5725609
- DOI: 10.1042/BSR20170926
PU.1-deficient mice are resistant to thioacetamide-induced hepatic fibrosis: PU.1 finely regulates Sirt1 expression via transcriptional promotion of miR-34a and miR-29c in hepatic stellate cells
Abstract
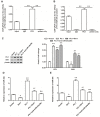
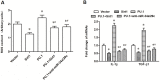
PU box binding protein (PU.1) is a critical transcription factor involved in many pathological processes. However, its exact role in activation of hepatic stellate cells (HSCs) and liver fibrosis was rarely reported. Here, we found that, in HSCs of PU.1+/- mice, Sirt1 mRNA expression was not changed but Sirt1 protein was significantly increased, suggesting its promoting role in Sirt1 translation. We then isolated HSCs from wild-type (WT) and PU.1+/- mice, and the pcDNA-PU.1 expression vector was transfected into PU.1+/- HSCs. We checked the levels of miR-34a and miR-29c, two Sirt1-targetting miRNAs, and protein levels of PU.1 and Sirt1. The results showed that miR-34a/-29c were significantly reduced and Sirt1 protein was increased in PU.1+/- HSCs, compared with WT HSCs. Besides, PU.1 overexpression inversed the reduction in miR-34a/-29c levels and the increase in Sirt1 protein in both PU.1+/- HSCs and WT HSCs. Additionally, ChIP-quantitive real-time PCR (qPCR) assay comfirmed that PU.1 was directly bound to both the promoter regions of miR-34a and miR-29c Importantly, PU.1 overexpression promoted the proliferation, migration, activation, oxidative stress and inflammatory response in WT HSCs, while the promotion could be inversed by either overexpression of Sirt1 or inhibition of miR-34a/-29c Moreover, animal model of liver fibrosis was established by intraperitoneal injections of thioacetamide (TAA) in WT and PU.1+/- mice, respectively. Compared with the WT mice, PU.1+/- mice displayed a lower fibrotic score, less collagen content, better liver function, and lower levels of oxidative stress and inflammatory response. In conclusion, PU.1 suppresses Sirt1 translation via transcriptional promotion of miR-34a/-29c, thus promoting Sirt1-mediated HSC activation and TAA-induced hepatic fibrosis.
Keywords: PU.1; hepatic fibrosis; hepatic stellate cell activation; miR-34a/-29c; sirtuins.
© 2017 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures





References
-
- Bataller R. and Brenner D.A. (2001) Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin. Liver Dis. 21, 437–451 - PubMed
-
- Zhao W., Zhang L., Yin Y. et al. (2011) Activated hepatic stellate cells promote hepatocellular carcinoma development in immunocompetent mice. Int. J. Cancer 129, 2651–2661 - PubMed
-
- Bordone L. and Guarente L. (2005) Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat. Rev. Mol. Cell Biol. 6, 298–305 - PubMed
-
- Milner A.J. (2011) Induction of apoptosis by inhibition of sirtuin SIRT1 expression, 7863436. U.S. Pat.
-
- Prozorovski T., Schulze-Topphoff U., Glumm R. et al. (2008) Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat. Cell Biol. 10, 385–394 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

