Atypical activation of dendritic cells by Plasmodium falciparum
- PMID: 29162686
- PMCID: PMC5724257
- DOI: 10.1073/pnas.1708383114
Atypical activation of dendritic cells by Plasmodium falciparum
Abstract
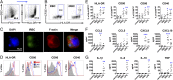
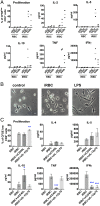
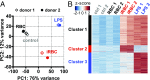
Dendritic cells (DCs) are activated by pathogens to initiate and shape immune responses. We found that the activation of DCs by Plasmodium falciparum, the main causative agent of human malaria, induces a highly unusual phenotype by which DCs up-regulate costimulatory molecules and secretion of chemokines, but not of cytokines typical of inflammatory responses (IL-1β, IL-6, IL-10, TNF). Similar results were obtained with DCs obtained from malaria-naïve US donors and malaria-experienced donors from Mali. Contact-dependent cross-talk between the main DC subsets, plasmacytoid and myeloid DCs (mDCs) was necessary for increased chemokine and IFN-α secretion in response to the parasite. Despite the absence of inflammatory cytokine secretion, mDCs incubated with P. falciparum-infected erythrocytes activated antigen-specific naïve CD4+ T cells to proliferate and secrete Th1-like cytokines. This unexpected response of human mDCs to P. falciparum exhibited a transcriptional program distinct from a classical LPS response, pointing to unique P. falciparum-induced activation pathways that may explain the uncharacteristic immune response to malaria.
Keywords: Plasmodium falciparum; activation; cytokines; dendritic cells; malaria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- World Health Organization (2016) World Malaria Report 2016 (World Health Organization, Geneva)
-
- Stanisic DI, Barry AE, Good MF. Escaping the immune system: How the malaria parasite makes vaccine development a challenge. Trends Parasitol. 2013;29:612–622. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Banchereau J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

