Outcomes of patients with sarcoma enrolled in clinical trials of pazopanib combined with histone deacetylase, mTOR, Her2, or MEK inhibitors
- PMID: 29162825
- PMCID: PMC5698336
- DOI: 10.1038/s41598-017-13114-8
Outcomes of patients with sarcoma enrolled in clinical trials of pazopanib combined with histone deacetylase, mTOR, Her2, or MEK inhibitors
Abstract
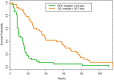
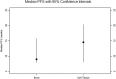
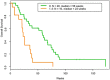
Pazopanib is US FDA approved for the treatment of advanced soft tissue sarcomas. All patients with this disease ultimately develop resistance to therapy. Mechanisms of resistance include activation of the mTOR, histone deacetylase (HDAC), MAPK, and ERBB4 pathways. We hypothesized that combining pazopanib with other targeted agents inhibiting these pathways would increase response rates. We retrospectively evaluated the safety and efficacy of pazopanib plus vorinostat, everolimus, lapatinib or trastuzumab, and MEK inhibitor in patients with advanced sarcoma. The Cancer Geneome Atlas (TCGA) data was analyzed for HDAC, PI3K, HER2, and MAPK/RAS/RAF gene alterations from sarcoma TCGA. Of the 44 advanced sarcoma patients in these trials, 27 (61%) were male; 18 (41%) had bone sarcoma, and 26 (59%) had soft tissue sarcoma. Best response was partial response (PR) in four patients [(overall response rate (ORR) = 9%, 95% confidence interval [CI] 3% to 22%)]. The median progression-free survival (PFS) for all patients was 9.6 weeks (95% CI 8.0 to 15.7 weeks). Analysis of TCGA data revealed HDAC, PI3K, HER2, and MAPK/RAS/RAF gene alterations in 112/243 (46%) of patients predominantly HDAC1-11 (41%) alterations. Pazopanib combinations did demonstrate safety in combination with other agents. TCGA data suggests further evaluation of epigenetic pathway inhibitors in sarcoma.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

