Single-molecule visualization of conformational changes and substrate transport in the vitamin B12 ABC importer BtuCD-F
- PMID: 29162829
- PMCID: PMC5698293
- DOI: 10.1038/s41467-017-01815-7
Single-molecule visualization of conformational changes and substrate transport in the vitamin B12 ABC importer BtuCD-F
Abstract
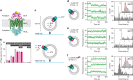
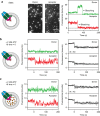
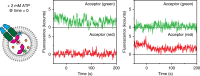
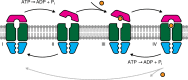
ATP-binding cassette (ABC) transporters form the largest class of active membrane transport proteins. Binding and hydrolysis of ATP by their highly conserved nucleotide-binding domains drive conformational changes of the complex that mediate transport of substrate across the membrane. The vitamin B12 importer BtuCD-F in Escherichia coli is an extensively studied model system. The periplasmic soluble binding protein BtuF binds the ligand; the transmembrane and ATPase domains BtuCD mediate translocation. Here we report the direct observation at the single-molecule level of ATP, vitamin B12 and BtuF-induced events in the transporter complex embedded in liposomes. Single-molecule fluorescence imaging techniques reveal that membrane-embedded BtuCD forms a stable complex with BtuF, regardless of the presence of ATP and vitamin B12. We observe that a vitamin B12 molecule remains bound to the complex for tens of seconds, during which several ATP hydrolysis cycles can take place, before it is being transported across the membrane.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

