Dysbiosis of the microbiome in gastric carcinogenesis
- PMID: 29162924
- PMCID: PMC5698432
- DOI: 10.1038/s41598-017-16289-2
Dysbiosis of the microbiome in gastric carcinogenesis
Abstract
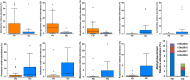
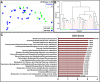
The gastric microbiome has been proposed as an etiological factor in gastric carcinogenesis. We compared the gastric microbiota in subjects presenting with gastric cancer (GC, n = 12) and controls (functional dyspepsia (FD), n = 20) from a high GC risk population in Singapore and Malaysia. cDNA from 16S rRNA transcripts were amplified (515F-806R) and sequenced using Illumina MiSeq 2 × 250 bp chemistry. Increased richness and phylogenetic diversity but not Shannon's diversity was found in GC as compared to controls. nMDS clustered GC and FD subjects separately, with PERMANOVA confirming a significant difference between the groups. H. pylori serological status had a significant impact on gastric microbiome α-diversity and composition. Several bacterial taxa were enriched in GC, including Lactococcus, Veilonella, and Fusobacteriaceae (Fusobacterium and Leptotrichia). Prediction of bacterial metabolic contribution indicated that serological status had a significant impact on metabolic function, while carbohydrate digestion and pathways were enriched in GC. Our findings highlight three mechanisms of interest in GC, including enrichment of pro-inflammatory oral bacterial species, increased abundance of lactic acid producing bacteria, and enrichment of short chain fatty acid production pathways.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
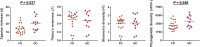


References
-
- Ferlay, J. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide. IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer (2013).
-
- Lertpiriyapong K, et al. Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut. 2014;63:54–63. doi: 10.1136/gutjnl-2013-305178. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

