Resveratrol Attenuates Early Brain Injury after Experimental Subarachnoid Hemorrhage via Inhibition of NLRP3 Inflammasome Activation
- PMID: 29163015
- PMCID: PMC5675880
- DOI: 10.3389/fnins.2017.00611
Resveratrol Attenuates Early Brain Injury after Experimental Subarachnoid Hemorrhage via Inhibition of NLRP3 Inflammasome Activation
Abstract
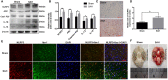
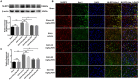
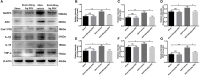
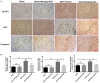
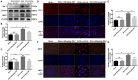
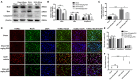
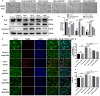
Previous studies have demonstrated resveratrol (RSV) has beneficial effects in early brain injury (EBI) after subarachnoid hemorrhage (SAH). However, the beneficial effects of RSV and the underlying mechanisms have not been clearly identified. The nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activation plays a crucial role in the EBI pathogenesis. The aim of this study was to investigate the role of RSV on the NLRP3 inflammasome signaling pathway and EBI in rats after SAH. A prechiasmatic cistern injection model was established in rats, and the primary cultured cortical neurons were stimulated with oxyhemoglobin (oxyHb) to induce SAH in vitro. It showed that the NLRP3 inflammasome components, including NLRP3, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), caspase-1, mature interleukin-1β (IL-1β), and interleukin-18 (IL-18) were upregulated after SAH, and the enhanced NLRP3 after SAH was mainly located in microglia. Treatment with 60 or 90 mg/kg RSV after SAH dramatically inhibited the expression of NLRP3, but there was no significant difference in the expression of NLRP3 between the SAH + 60 mg/kg RSV and SAH + 90 mg/kg RSV groups. In addition, treatment with 30 mg/kg RSV did not significantly reduced the expression of NLRP3. We next evaluated the neuroprotective effects of RSV against SAH. We determined that SAH-induced NLRP3 inflammasome activation was significantly inhibited in the SAH + 60 mg/kg RSV group. Meanwhile, 60 mg/kg RSV administration could markedly inhibit microglia activation and neutrophils infiltration after SAH. Concomitant with the decreased cerebral inflammation, RSV evidently reduced cortical apoptosis, brain edema, and neurobehavioral impairment after SAH. In vitro experiments, RSV treatment also clearly protected primary cortical neurons against oxyHb insults, including reduced the proportion of neuronal apoptosis, alleviated neuronal degeneration, and improved cell viabilities. These in vitro data further confirm that RSV has an efficient neuroprotection against SAH. Taken together, these in vivo and in vitro findings suggested RSV could protect against EBI after SAH, at least partially via inhibiting NLRP3 inflammasome signaling pathway.
Keywords: NLRP3; early brain injury; inflammation; resveratrol; subarachnoid hemorrhage.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

