Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System
- PMID: 29163019
- PMCID: PMC5681992
- DOI: 10.3389/fnins.2017.00617
Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System
Abstract
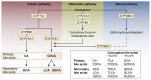
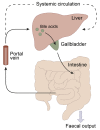
Bile acids are best known as detergents involved in the digestion of lipids. In addition, new data in the last decade have shown that bile acids also function as gut hormones capable of influencing metabolic processes via receptors such as FXR (farnesoid X receptor) and TGR5 (Takeda G protein-coupled receptor 5). These effects of bile acids are not restricted to the gastrointestinal tract, but can affect different tissues throughout the organism. It is still unclear whether these effects also involve signaling of bile acids to the central nervous system (CNS). Bile acid signaling to the CNS encompasses both direct and indirect pathways. Bile acids can act directly in the brain via central FXR and TGR5 signaling. In addition, there are two indirect pathways that involve intermediate agents released upon interaction with bile acids receptors in the gut. Activation of intestinal FXR and TGR5 receptors can result in the release of fibroblast growth factor 19 (FGF19) and glucagon-like peptide 1 (GLP-1), both capable of signaling to the CNS. We conclude that when plasma bile acids levels are high all three pathways may contribute in signal transmission to the CNS. However, under normal physiological circumstances, the indirect pathway involving GLP-1 may evoke the most substantial effect in the brain.
Keywords: CNS; FGF19; FXR; GLP-1; TGR5; bile acids; brain.
Figures

References
-
- Abbott C. R., Monteiro M., Small C. J., Sajedi A., Smith K. L., Parkinson J. R., et al. . (2005). The inhibitory effects of peripheral administration of peptide YY 3–36 and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal–brainstem–hypothalamic pathway. Brain Res. 1044, 127–131. 10.1016/j.brainres.2005.03.011 - DOI - PubMed
-
- Abe T., Kakyo M., Sakagami H., Tokui T., Nishio T., Tanemoto M., et al. . (1998). Molecular characterization and tissue distribution of a new organic anion transporter subtype (oatp3) that transports thyroid hormones and taurocholate and comparison with oatp2. J. Biol. Chem. 273, 22395–22401. 10.1074/jbc.273.35.22395 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

