Partial Aminoglycoside Lesions in Vestibular Epithelia Reveal Broad Sensory Dysfunction Associated with Modest Hair Cell Loss and Afferent Calyx Retraction
- PMID: 29163044
- PMCID: PMC5663721
- DOI: 10.3389/fncel.2017.00331
Partial Aminoglycoside Lesions in Vestibular Epithelia Reveal Broad Sensory Dysfunction Associated with Modest Hair Cell Loss and Afferent Calyx Retraction
Abstract
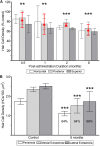
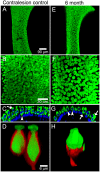
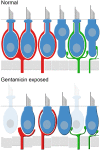
Although the effects of aminoglycoside antibiotics on hair cells have been investigated for decades, their influences on the dendrites of primary afferent neurons have not been widely studied. This is undoubtedly due to the difficulty in disassociating pathology to dendritic processes from that resulting from loss of the presynaptic hair cell. This was overcome in the present investigation through development of a preparation using Chinchilla laniger that enabled direct perilymphatic infusion. Through this strategy we unmasked gentamicin's potential effects on afferent calyces. The pathophysiology of the vestibular neuroepithelia after post-administration durations of 0.5 through 6 months was assessed using single-neuron electrophysiology, immunohistochemistry, and confocal microscopy. Hair cell densities within cristae central zones (0.5-, 1-, 2-, and 6-months) and utricle peri- and extrastriola (6-months) regions were determined, and damage to calretinin-immunoreactive calyces was quantified. Gentamicin-induced hair cell loss exhibited a profile that reflected elimination of a most-sensitive group by 0.5-months post-administration (18.2%), followed by loss of a second group (20.6%) over the subsequent 5.5 months. The total hair cell loss with this gentamicin dose (approximately 38.8%) was less than the estimated fraction of type I hair cells in the chinchilla's crista central zone (approximately 60%), indicating that viable type I hair cells remained. Extensive lesions to afferent calyces were observed at 0.5-months, though stimulus-evoked modulation was intact at this post-administration time. Widespread compromise to calyx morphology and severe attenuation of stimulus-evoked afferent discharge modulation was found at 1 month post-administration, a condition that persisted in preparations examined through the 6-month post-administration interval. Spontaneous discharge was robust at all post-administration intervals. All calretinin-positive calyces had retracted at 2 and 6 months post-administration. We found no evidence of morphologic or physiologic recovery. These results indicate that gentamicin-induced partial lesions to vestibular epithelia include hair cell loss (ostensibly reflecting an apoptotic effect) that is far less extensive than the compromise to stimulus-evoked afferent discharge modulation and retraction of afferent calyces (reflecting non-apoptotic effects). Additionally, calyx retraction cannot be completely accounted for by loss of type I hair cells, supporting the possibility for direct action of gentamicin on the afferent dendrite.
Keywords: ototoxicity; primary afferent; spontaneous discharge; stimulus–response coherence.
Figures









Similar articles
-
The vestibular nerve of the chinchilla. I. Peripheral innervation patterns in the horizontal and superior semicircular canals.J Neurophysiol. 1988 Jul;60(1):167-81. doi: 10.1152/jn.1988.60.1.167. J Neurophysiol. 1988. PMID: 3404215
-
Persistent and resurgent Na+ currents in vestibular calyx afferents.J Neurophysiol. 2020 Aug 1;124(2):510-524. doi: 10.1152/jn.00124.2020. Epub 2020 Jul 15. J Neurophysiol. 2020. PMID: 32667253 Free PMC article.
-
Expression of hyperpolarization-activated current (Ih) in zonally defined vestibular calyx terminals of the crista.J Neurophysiol. 2023 Jun 1;129(6):1468-1481. doi: 10.1152/jn.00135.2023. Epub 2023 May 17. J Neurophysiol. 2023. PMID: 37198134 Free PMC article.
-
Morphophysiological and ultrastructural studies in the mammalian cristae ampullares.Hear Res. 1990 Nov;49(1-3):89-102. doi: 10.1016/0378-5955(90)90097-9. Hear Res. 1990. PMID: 2292511 Review.
-
Simultaneous Dual Recordings From Vestibular Hair Cells and Their Calyx Afferents Demonstrate Multiple Modes of Transmission at These Specialized Endings.Front Neurol. 2022 Jul 11;13:891536. doi: 10.3389/fneur.2022.891536. eCollection 2022. Front Neurol. 2022. PMID: 35899268 Free PMC article. Review.
Cited by
-
Vestibular Injury After Low-Intensity Blast Exposure.Front Neurol. 2018 May 14;9:297. doi: 10.3389/fneur.2018.00297. eCollection 2018. Front Neurol. 2018. PMID: 29867715 Free PMC article.
-
Mechanisms of Aminoglycoside- and Cisplatin-Induced Ototoxicity.Am J Audiol. 2021 Oct 11;30(3S):887-900. doi: 10.1044/2021_AJA-21-00006. Epub 2021 Aug 20. Am J Audiol. 2021. PMID: 34415784 Free PMC article. Review.
-
The vestibular calyceal junction is dismantled following subchronic streptomycin in rats and sensory epithelium stress in humans.Arch Toxicol. 2023 Jul;97(7):1943-1961. doi: 10.1007/s00204-023-03518-z. Epub 2023 May 17. Arch Toxicol. 2023. PMID: 37195449 Free PMC article.
-
Pharmacological regeneration of sensory hair cells restores afferent innervation and vestibular function.J Clin Invest. 2024 Sep 24;134(22):e181201. doi: 10.1172/JCI181201. J Clin Invest. 2024. PMID: 39316439 Free PMC article.
-
Direct current effects on afferent and hair cell to elicit natural firing patterns.iScience. 2021 Feb 20;24(3):102205. doi: 10.1016/j.isci.2021.102205. eCollection 2021 Mar 19. iScience. 2021. PMID: 33748701 Free PMC article.
References
-
- Baird R. A., Desmadryl G., Fernandez C., Goldberg J. M. (1988). The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J. Neurophysiol. 60 182–203. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

