The Lesioned Spinal Cord Is a "New" Spinal Cord: Evidence from Functional Changes after Spinal Injury in Lamprey
- PMID: 29163065
- PMCID: PMC5681538
- DOI: 10.3389/fncir.2017.00084
The Lesioned Spinal Cord Is a "New" Spinal Cord: Evidence from Functional Changes after Spinal Injury in Lamprey
Abstract
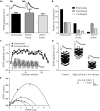
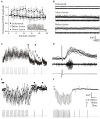
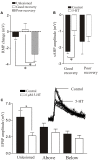
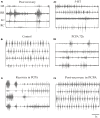
Finding a treatment for spinal cord injury (SCI) focuses on reconnecting the spinal cord by promoting regeneration across the lesion site. However, while regeneration is necessary for recovery, on its own it may not be sufficient. This presumably reflects the requirement for regenerated inputs to interact appropriately with the spinal cord, making sub-lesion network properties an additional influence on recovery. This review summarizes work we have done in the lamprey, a model system for SCI research. We have compared locomotor behavior (swimming) and the properties of descending inputs, locomotor networks, and sensory inputs in unlesioned animals and animals that have received complete spinal cord lesions. In the majority (∼90%) of animals swimming parameters after lesioning recovered to match those in unlesioned animals. Synaptic inputs from individual regenerated axons also matched the properties in unlesioned animals, although this was associated with changes in release parameters. This suggests against any compensation at these synapses for the reduced descending drive that will occur given that regeneration is always incomplete. Compensation instead seems to occur through diverse changes in cellular and synaptic properties in locomotor networks and proprioceptive systems below, but also above, the lesion site. Recovery of locomotor performance is thus not simply the reconnection of the two sides of the spinal cord, but reflects a distributed and varied range of spinal cord changes. While locomotor network changes are insufficient on their own for recovery, they may facilitate locomotor outputs by compensating for the reduction in descending drive. Potentiated sensory feedback may in turn be a necessary adaptation that monitors and adjusts the output from the "new" locomotor network. Rather than a single aspect, changes in different components of the motor system and their interactions may be needed after SCI. If these are general features, and where comparisons with mammalian systems can be made effects seem to be conserved, improving functional recovery in higher vertebrates will require interventions that generate the optimal spinal cord conditions conducive to recovery. The analyses needed to identify these conditions are difficult in the mammalian spinal cord, but lower vertebrate systems should help to identify the principles of the optimal spinal cord response to injury.
Keywords: lamprey; neuromodulation; plasticity; regeneration; spinal cord injury.
Figures





References
-
- Ashby W. (1958). Requisite variety and its implications for the control of complex systems. Cybernetics 1 83–99.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

