The Paravascular Pathway for Brain Waste Clearance: Current Understanding, Significance and Controversy
- PMID: 29163074
- PMCID: PMC5681909
- DOI: 10.3389/fnana.2017.00101
The Paravascular Pathway for Brain Waste Clearance: Current Understanding, Significance and Controversy
Abstract
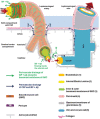
The paravascular pathway, also known as the "glymphatic" pathway, is a recently described system for waste clearance in the brain. According to this model, cerebrospinal fluid (CSF) enters the paravascular spaces surrounding penetrating arteries of the brain, mixes with interstitial fluid (ISF) and solutes in the parenchyma, and exits along paravascular spaces of draining veins. Studies have shown that metabolic waste products and solutes, including proteins involved in the pathogenesis of neurodegenerative diseases such as amyloid-beta, may be cleared by this pathway. Consequently, a growing body of research has begun to explore the association between glymphatic dysfunction and various disease states. However, significant controversy exists in the literature regarding both the direction of waste clearance as well as the anatomical space in which the waste-fluid mixture is contained. Some studies have found no evidence of interstitial solute clearance along the paravascular space of veins. Rather, they demonstrate a perivascular pathway in which waste is cleared from the brain along an anatomically distinct perivascular space in a direction opposite to that of paravascular flow. Although possible explanations have been offered, none have been able to fully reconcile the discrepancies in the literature, and many questions remain. Given the therapeutic potential that a comprehensive understanding of brain waste clearance pathways might offer, further research and clarification is highly warranted.
Keywords: amyloid-beta; brain waste clearance; glymphatic system; paravascular pathway; perivascular pathway.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

