A Sensitivity Analysis of an Inverted Pendulum Balance Control Model
- PMID: 29163116
- PMCID: PMC5664365
- DOI: 10.3389/fncom.2017.00099
A Sensitivity Analysis of an Inverted Pendulum Balance Control Model
Abstract
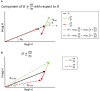
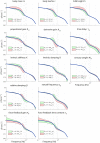
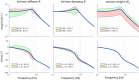
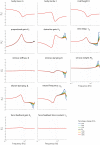
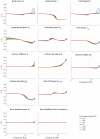
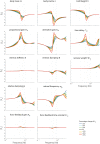
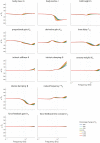
Balance control models are used to describe balance behavior in health and disease. We identified the unique contribution and relative importance of each parameter of a commonly used balance control model, the Independent Channel (IC) model, to identify which parameters are crucial to describe balance behavior. The balance behavior was expressed by transfer functions (TFs), representing the relationship between sensory perturbations and body sway as a function of frequency, in terms of amplitude (i.e., magnitude) and timing (i.e., phase). The model included an inverted pendulum controlled by a neuromuscular system, described by several parameters. Local sensitivity of each parameter was determined for both the magnitude and phase using partial derivatives. Both the intrinsic stiffness and proportional gain shape the magnitude at low frequencies (0.1-1 Hz). The derivative gain shapes the peak and slope of the magnitude between 0.5 and 0.9 Hz. The sensory weight influences the overall magnitude, and does not have any effect on the phase. The effect of the time delay becomes apparent in the phase above 0.6 Hz. The force feedback parameters and intrinsic stiffness have a small effect compared with the other parameters. All parameters shape the TF magnitude and phase and therefore play a role in the balance behavior. The sensory weight, time delay, derivative gain, and the proportional gain have a unique effect on the TFs, while the force feedback parameters and intrinsic stiffness contribute less. More insight in the unique contribution and relative importance of all parameters shows which parameters are crucial and critical to identify underlying differences in balance behavior between different patient groups.
Keywords: balance control model; frequency domain; human balance control; parameters; sensitivity analysis.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

