Functional Mitochondria in Health and Disease
- PMID: 29163365
- PMCID: PMC5675848
- DOI: 10.3389/fendo.2017.00296
Functional Mitochondria in Health and Disease
Abstract
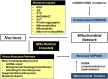
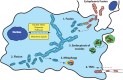
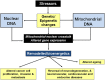
The ability to rapidly adapt cellular bioenergetic capabilities to meet rapidly changing environmental conditions is mandatory for normal cellular function and for cancer progression. Any loss of this adaptive response has the potential to compromise cellular function and render the cell more susceptible to external stressors such as oxidative stress, radiation, chemotherapeutic drugs, and hypoxia. Mitochondria play a vital role in bioenergetic and biosynthetic pathways and can rapidly adjust to meet the metabolic needs of the cell. Increased demand is met by mitochondrial biogenesis and fusion of individual mitochondria into dynamic networks, whereas a decrease in demand results in the removal of superfluous mitochondria through fission and mitophagy. Effective communication between nucleus and mitochondria (mito-nuclear cross talk), involving the generation of different mitochondrial stress signals as well as the nuclear stress response pathways to deal with these stressors, maintains bioenergetic homeostasis under most conditions. However, when mitochondrial DNA (mtDNA) mutations accumulate and mito-nuclear cross talk falters, mitochondria fail to deliver critical functional outputs. Mutations in mtDNA have been implicated in neuromuscular and neurodegenerative mitochondriopathies and complex diseases such as diabetes, cardiovascular diseases, gastrointestinal disorders, skin disorders, aging, and cancer. In some cases, drastic measures such as acquisition of new mitochondria from donor cells occurs to ensure cell survival. This review starts with a brief discussion of the evolutionary origin of mitochondria and summarizes how mutations in mtDNA lead to mitochondriopathies and other degenerative diseases. Mito-nuclear cross talk, including various stress signals generated by mitochondria and corresponding stress response pathways activated by the nucleus are summarized. We also introduce and discuss a small family of recently discovered hormone-like mitopeptides that modulate body metabolism. Under conditions of severe mitochondrial stress, mitochondria have been shown to traffic between cells, replacing mitochondria in cells with damaged and malfunctional mtDNA. Understanding the processes involved in cellular bioenergetics and metabolic adaptation has the potential to generate new knowledge that will lead to improved treatment of many of the metabolic, degenerative, and age-related inflammatory diseases that characterize modern societies.
Keywords: mito-nuclear cross talk; mitochondrial DNA; mitochondrial DNA mutations; mitochondrial stress signals; mitochondrial transfer; mitochondriopathies; mitopeptides.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

