Evidence Suggesting Absence of Mitochondrial DNA Methylation
- PMID: 29163634
- PMCID: PMC5671948
- DOI: 10.3389/fgene.2017.00166
Evidence Suggesting Absence of Mitochondrial DNA Methylation
Abstract
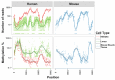
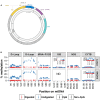
Methylation of nuclear genes encoding mitochondrial proteins participates in the regulation of mitochondria function. The existence of cytosine methylation in the mitochondrial genome is debated. To investigate whether mitochondrial DNA (mtDNA) is methylated, we used both targeted- and whole mitochondrial genome bisulfite sequencing in cell lines and muscle tissue from mouse and human origin. While unconverted cytosines were detected in some portion of the mitochondrial genome, their abundance was inversely associated to the sequencing depth, indicating that sequencing analysis can bias the estimation of mtDNA methylation levels. In intact mtDNA, few cytosines remained 100% unconverted. However, removal of supercoiled structures of mtDNA with the restriction enzyme BamHI prior to bisulfite sequencing decreased cytosine unconversion rate to <1.5% at all the investigated regions: D-loop, tRNA-F+12S, 16S, ND5 and CYTB, suggesting that mtDNA supercoiled structure blocks the access to bisulfite conversion. Here, we identified an artifact of mtDNA bisulfite sequencing that can lead to an overestimation of mtDNA methylation levels. Our study supports that cytosine methylation is virtually absent in mtDNA.
Keywords: DNA methylation; bisulfite sequencing; epigenetics; mitochondria; whole genome bisulfite sequencing.
Figures

References
-
- Anon (2017). Rdatatable/data.table. 1–101. Available at: http://r-datatable.com
LinkOut - more resources
Full Text Sources
Other Literature Sources

