Inhibition of autoimmune Th17 cell responses by pain killer ketamine
- PMID: 29163764
- PMCID: PMC5685685
- DOI: 10.18632/oncotarget.18324
Inhibition of autoimmune Th17 cell responses by pain killer ketamine
Abstract
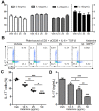
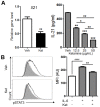
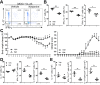
Ketamine is widely used in animals and humans as a systemic anesthetic. Although several immune-modulatory functions of ketamine have been reported, the effects of ketamine on the differentiation of Th17 cell are unknown. We found that ketamine significantly diminished the frequency of IL-17-producers among CD4+ T cells stimulated under Th17-skewing conditions. Mechanistic studies showed that ketamine had little effect on the production of Th17-inducing cytokines by dendritic cells and the proliferation of T cells in response to anti-CD3; however it significantly hampered IL-21 expression as well as STAT3 phosphorylation in T cells upon IL-6 stimulation. Moreover, MOG-reactive CD4+ T cells expanded in the presence of ketamine produced reduced amounts of Th17 cytokines, leading to diminished EAE severity when transferred into TCRβ-deficient mice in comparison to those treated with vehicle. These findings demonstrate that ketamine suppresses autoimmune Th17 cell responses by inhibiting the differentiation as well as the reactivation of Th17 cells.
Keywords: IL-21; Immune response; Immunity; Immunology and Microbiology Section; STAT3; Th17 cell; autoimmunity; ketamine.
Conflict of interest statement
CONFLICTS OF INTEREST None declared.
Figures



Similar articles
-
Mannan-conjugated myelin peptides prime non-pathogenic Th1 and Th17 cells and ameliorate experimental autoimmune encephalomyelitis.Exp Neurol. 2015 May;267:254-67. doi: 10.1016/j.expneurol.2014.10.019. Epub 2014 Oct 30. Exp Neurol. 2015. PMID: 25447934
-
Green tea EGCG, T cells, and T cell-mediated autoimmune diseases.Mol Aspects Med. 2012 Feb;33(1):107-18. doi: 10.1016/j.mam.2011.10.001. Epub 2011 Oct 14. Mol Aspects Med. 2012. PMID: 22020144 Review.
-
A novel human STAT3 mutation presents with autoimmunity involving Th17 hyperactivation.Oncotarget. 2015 Aug 21;6(24):20037-42. doi: 10.18632/oncotarget.5042. Oncotarget. 2015. PMID: 26343524 Free PMC article.
-
Invariant NKT cells producing IL-4 or IL-10, but not IFN-gamma, inhibit the Th1 response in experimental autoimmune encephalomyelitis, whereas none of these cells inhibits the Th17 response.J Immunol. 2011 Jun 15;186(12):6815-21. doi: 10.4049/jimmunol.1003916. Epub 2011 May 13. J Immunol. 2011. PMID: 21572032
-
Regulatory B and T cell responses in patients with autoimmune thyroid disease and healthy controls.Dan Med J. 2016 Feb;63(2):B5177. Dan Med J. 2016. PMID: 26836805 Review.
Cited by
-
Role of Inflammatory Mechanisms in Major Depressive Disorder: From Etiology to Potential Pharmacological Targets.Cells. 2024 Feb 28;13(5):423. doi: 10.3390/cells13050423. Cells. 2024. PMID: 38474387 Free PMC article. Review.
-
Pharmaceutical therapeutics for articular regeneration and restoration: state-of-the-art technology for screening small molecular drugs.Cell Mol Life Sci. 2021 Dec;78(24):8127-8155. doi: 10.1007/s00018-021-03983-8. Epub 2021 Nov 16. Cell Mol Life Sci. 2021. PMID: 34783870 Free PMC article. Review.
-
Sepsis impedes EAE disease development and diminishes autoantigen-specific naive CD4 T cells.Elife. 2020 Nov 16;9:e55800. doi: 10.7554/eLife.55800. Elife. 2020. PMID: 33191915 Free PMC article.
-
Ketamine in Bipolar Disorder: A Review.Neuropsychiatr Dis Treat. 2020 Nov 12;16:2707-2717. doi: 10.2147/NDT.S282208. eCollection 2020. Neuropsychiatr Dis Treat. 2020. PMID: 33209026 Free PMC article. Review.
-
Th17 Cells in Depression: Are They Crucial for the Antidepressant Effect of Ketamine?Front Pharmacol. 2021 Apr 15;12:649144. doi: 10.3389/fphar.2021.649144. eCollection 2021. Front Pharmacol. 2021. PMID: 33935753 Free PMC article. Review.
References
-
- Sekandarzad MW, van Zundert AA, Lirk PB, Doornebal CW, Hollmann MW. Perioperative Anesthesia Care and Tumor Progression. Anesth Analg. 2017;124:1697–708. - PubMed
-
- Ji FH, Wang YL, Yang JP. Effects of propofol anesthesia and sevoflurane anesthesia on the differentiation of human T-helper cells during surgery. Chin Med J (Engl) 2011;124:525–29. - PubMed
-
- Morgan CJ, Curran HV, Independent Scientific Committee on Drugs Ketamine use: a review. Addiction. 2012;107:27–38. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

