Displacement and hybridization reactions in aptamer-functionalized hydrogels for biomimetic protein release and signal transduction
- PMID: 29163881
- PMCID: PMC5672785
- DOI: 10.1039/c7sc03023a
Displacement and hybridization reactions in aptamer-functionalized hydrogels for biomimetic protein release and signal transduction
Abstract
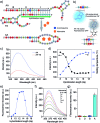
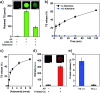
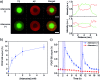
A variety of hydrogels have been synthesized for controlling the release of signaling molecules in applications such as drug delivery and regenerative medicine. However, it remains challenging to synthesize hydrogels with the ability to control the release of signaling molecules sequentially or periodically under physiological conditions as living cells do in response to the variation of metabolism. The purpose of this work was to study a novel biomimetic hydrogel system with the ability of recapitulating the procedure of cellular signal transduction and controlling the sequential release of signaling molecules under physiological conditions. In the presence of a small chemical, the signaling molecule is regulated to change from a DNA-bound state to a free state and the freed signaling molecule is able to regulate intracellular signal transduction and cell migration. Moreover, periodic exposure of the hydrogel system to the small chemical leads to sequential protein release. Since signaling molecules are important for every activity of the cell, this hydrogel system holds potential as a metabolism-responsive platform for controlled release of signaling molecules and cell regulation in various applications.
Figures


References
-
- Hoffman A. S. Adv. Drug Delivery Rev. 2002;54:3–12. - PubMed
- Lutolf M. P., Hubbell J. A. Nat. Biotechnol. 2005;23:47–55. - PubMed
- Yu L., Ding J. D. Chem. Soc. Rev. 2008;37:1473–1481. - PubMed
- Li J., Mo L. T., Lu C. H., Fu T., Yang H. H., Tan W. H. Chem. Soc. Rev. 2016;45:1410–1431. - PMC - PubMed
- Lin C. C. RSC Adv. 2015;5:39844–39853. - PMC - PubMed
-
- Huang F. J., Liao W. C., Sohn Y. S., Nechushtai R., Lu C. H., Willner I. J. Am. Chem. Soc. 2016;138:8936–8945. - PubMed
- Hu Y. W., Guo W. W., Kahn J. S., Aleman-Garcia M. A., Willner I. Angew. Chem., Int. Ed. 2016;55:4210–4214. - PubMed
- Cheng E. J., Xing Y. Z., Chen P., Yang Y., Sun Y. W., Zhou D. J., Xu L. J., Fan Q. H., Liu D. S. Angew. Chem., Int. Ed. 2009;48:7660–7663. - PubMed
-
- Zhang Z. Y., Chen N. C., Li S. H., Battig M. R., Wang Y. J. Am. Chem. Soc. 2012;134:15716–15719. - PubMed
-
- Tomatsu I., Peng K., Kros A. Adv. Drug Delivery Rev. 2011;63:1257–1266. - PubMed
- Fomina N., Sankaranarayanan J., Almutairi A. Adv. Drug Delivery Rev. 2012;64:1005–1020. - PMC - PubMed
- Yan B., Boyer J. C., Habault D., Branda N. R., Zhao Y. J. Am. Chem. Soc. 2012;134:16558–16561. - PubMed
- Pasparakis G., Manouras T., Vamvakaki M., Argitis P. Nat. Commun. 2014;5:3623. - PMC - PubMed
- Liu J. W. Soft Matter. 2011;7:6757–6767.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

