Manganese complex-catalyzed oxidation and oxidative kinetic resolution of secondary alcohols by hydrogen peroxide
- PMID: 29163900
- PMCID: PMC5676093
- DOI: 10.1039/c7sc00891k
Manganese complex-catalyzed oxidation and oxidative kinetic resolution of secondary alcohols by hydrogen peroxide
Abstract
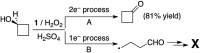
The highly efficient catalytic oxidation and oxidative kinetic resolution (OKR) of secondary alcohols has been achieved using a synthetic manganese catalyst with low loading and hydrogen peroxide as an environmentally benign oxidant in the presence of a small amount of sulfuric acid as an additive. The product yields were high (up to 93%) for alcohol oxidation and the enantioselectivity was excellent (>90% ee) for the OKR of secondary alcohols. Mechanistic studies revealed that alcohol oxidation occurs via hydrogen atom (H-atom) abstraction from an α-CH bond of the alcohol substrate and a two-electron process by an electrophilic Mn-oxo species. Density functional theory calculations revealed the difference in reaction energy barriers for H-atom abstraction from the α-CH bonds of R- and S-enantiomers by a chiral high-valent manganese-oxo complex, supporting the experimental result from the OKR of secondary alcohols.
Figures



Similar articles
-
Ruthenium-catalyzed oxidative kinetic resolution of unactivated and activated secondary alcohols with air as the hydrogen acceptor at room temperature.Angew Chem Int Ed Engl. 2014 Mar 17;53(12):3178-82. doi: 10.1002/anie.201310426. Epub 2014 Feb 12. Angew Chem Int Ed Engl. 2014. PMID: 24519886
-
Hydrogen-atom abstraction reactions by manganese(V)- and manganese(IV)-oxo porphyrin complexes in aqueous solution.Chemistry. 2009 Nov 2;15(43):11482-9. doi: 10.1002/chem.200901362. Chemistry. 2009. PMID: 19810056
-
Bioinspired Manganese and Iron Complexes for Enantioselective Oxidation Reactions: Ligand Design, Catalytic Activity, and Beyond.Acc Chem Res. 2019 Aug 20;52(8):2370-2381. doi: 10.1021/acs.accounts.9b00285. Epub 2019 Jul 23. Acc Chem Res. 2019. PMID: 31333021
-
Manganese-Oxygen Intermediates in O-O Bond Activation and Hydrogen-Atom Transfer Reactions.Acc Chem Res. 2017 Nov 21;50(11):2706-2717. doi: 10.1021/acs.accounts.7b00343. Epub 2017 Oct 24. Acc Chem Res. 2017. PMID: 29064667 Review.
-
Advances in asymmetric oxidative kinetic resolution of racemic secondary alcohols catalyzed by chiral Mn(III) salen complexes.Chirality. 2017 Dec;29(12):798-810. doi: 10.1002/chir.22768. Epub 2017 Sep 29. Chirality. 2017. PMID: 28963733 Review.
Cited by
-
Influence of Cysteine 440 on the Active Site Properties of 3-Deoxy-d-Arabino-Heptulosonate 7-Phosphate Synthase in Mycobacterium tuberculosis (MtDAHPS).ACS Omega. 2023 Apr 11;8(16):14401-14409. doi: 10.1021/acsomega.2c07662. eCollection 2023 Apr 25. ACS Omega. 2023. PMID: 37125090 Free PMC article.
-
Synthesis and Structures of Ruthenium Carbonyl Complexes Bearing Pyridine-Alkoxide Ligands and Their Catalytic Activity in Alcohol Oxidation.Front Chem. 2019 Jun 4;7:394. doi: 10.3389/fchem.2019.00394. eCollection 2019. Front Chem. 2019. PMID: 31214574 Free PMC article.
-
Shaping of a Reactive Manganese Catalyst Enables Access to Polyfunctionalized Cyclohexanes via Enantioselective C(sp3)─H Bond Oxidation of 1,3-meso Diethers.Angew Chem Int Ed Engl. 2025 Jul 21;64(30):e202507755. doi: 10.1002/anie.202507755. Epub 2025 Jun 1. Angew Chem Int Ed Engl. 2025. PMID: 40392708 Free PMC article.
-
Solventless, selective and catalytic oxidation of primary, secondary and benzylic alcohols by a Merrifield resin supported molybdenum(vi) complex with H2O2 as an oxidant.RSC Adv. 2018 Oct 8;8(60):34491-34504. doi: 10.1039/c8ra05969a. eCollection 2018 Oct 4. RSC Adv. 2018. PMID: 35548632 Free PMC article.
-
Manganese(III) complexes stabilized with N-heterocyclic carbene ligands for alcohol oxidation catalysis.Dalton Trans. 2023 Jun 13;52(23):7992-8002. doi: 10.1039/d3dt01013a. Dalton Trans. 2023. PMID: 37223983 Free PMC article.
References
-
- Abu-Omar M. M., Loaiza A., Hontzeas N. Chem. Rev. 2005;105:2227. - PubMed
- Krebs C., Fujimori D. G., Walsh C. T., Bollinger Jr J. M. Acc. Chem. Res. 2007;40:484. - PMC - PubMed
- Nam W. Acc. Chem. Res. 2007;40:522. - PubMed
- Cook S. A., Borovik A. S. Acc. Chem. Res. 2015;48:2407. - PMC - PubMed
- Nam W. Acc. Chem. Res. 2015;48:2415. - PubMed
- Puri M., Que Jr L. Acc. Chem. Res. 2015;48:2443. - PMC - PubMed
- Ray K., Heims F., Schwalbe M., Nam W. Curr. Opin. Chem. Biol. 2015;25:159. - PubMed
- Engelmann X., Monte-Pérez I., Ray K. Angew. Chem., Int. Ed. 2016;55:7632. - PubMed
- Sahu S., Goldberg D. P. J. Am. Chem. Soc. 2016;138:11410. - PMC - PubMed
- Proshlyakov D. A., McCracken J., Hausinger R. P. J. Biol. Inorg. Chem. 2017;22:367. - PMC - PubMed
- Yosca T. H., Ledray A. P., Ngo J., Green M. T. J. Biol. Inorg. Chem. 2017;22:209. - PMC - PubMed
- Kal S., Que Jr L. J. Biol. Inorg. Chem. 2017;22:339. - PubMed
-
- Que Jr L., Tolman W. B. Nature. 2008;455:333. - PubMed
- Gopalaiah K. Chem. Rev. 2013;113:3248. - PubMed
- Saisaha P., de Boer J. W., Browne W. R. Chem. Soc. Rev. 2013;42:2059. - PubMed
- Gelalcha F. G. Adv. Synth. Catal. 2014;356:261.
- Chen Z., Yin G. Chem. Soc. Rev. 2015;44:1083. - PubMed
- Collins T. J., Ryabov A. D. Chem. Rev. 2017;117:9140. - PubMed
-
- Codola Z., Lloret-Fillol J., Costas M. Prog. Inorg. Chem. 2014;59:447.
- Cussó O., Ribas X., Costas M. Chem. Commun. 2015;51:14285. - PubMed
- Olivo G., Cussó O., Costas M. Chem.–Asian J. 2016;11:3148. - PubMed
- Canta M., Rodríguez M., Costas M. Top. Curr. Chem. 2016;372:27. - PubMed
- Olivo G., Cussó O., Borrell M., Costas M. J. Biol. Inorg. Chem. 2017;22:425. - PubMed
- Milan M., Bietti M., Costas M. ACS Cent. Sci. 2017;3:196. - PMC - PubMed
-
- Oloo W. N., Que Jr L. Acc. Chem. Res. 2015;48:2612. - PubMed
- Oloo W. N., Meier K. K., Wang Y., Shaik S., Münck E., Que Jr L. Nat. Commun. 2014;5:3046. - PubMed
- Wang Y., Janardanan D., Usharani D., Han K., Que Jr L., Shaik S. ACS Catal. 2013;3:1334.
- Feng Y., England J., Que Jr L. ACS Catal. 2011;1:1035.
-
- Talsi E. P., Bryliakov K. P. Coord. Chem. Rev. 2012;256:1418.
- Bryliakov K. P., Talsi E. P. Coord. Chem. Rev. 2014;276:73.
- Ottenbacher R. V., Talsi E. P., Bryliakov K. P. Molecules. 2016;21:1454. - PMC - PubMed
- Ottenbacher R. V., Talsi E. P., Bryliakov K. P. Catal. Today. 2016;278:30.
- Zima A. M., Lyakin O. Y., Ottenbacher R. V., Bryliakov K. P., Talsi E. P. ACS Catal. 2017;7:60.
- Bryliakov K. P. Chem. Rev. 2017;117:11406. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

