Hyperfine adjustment of flexible pore-surface pockets enables smart recognition of gas size and quadrupole moment
- PMID: 29163911
- PMCID: PMC5676252
- DOI: 10.1039/c7sc03067c
Hyperfine adjustment of flexible pore-surface pockets enables smart recognition of gas size and quadrupole moment
Abstract
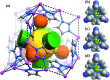
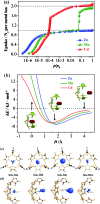
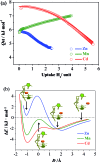
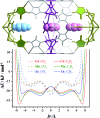
The pore size and framework flexibility of hosts are of vital importance for molecular recognition and related applications, but accurate control of these parameters is very challenging. We use the slight difference of metal ion size to achieve continuous hundredth-nanometer pore-size adjustments and drastic flexibility modulations in an ultramicroporous metal-organic framework, giving controllable N2 adsorption isotherm steps, unprecedented/reversed loading-dependence of H2 adsorption enthalpy, quadrupole-moment sieving of C2H2/CO2, and an exceptionally high working capacity for C2H2 storage under practical conditions (98 times that of an empty cylinder). In situ single-crystal X-ray diffraction measurements and multilevel computational simulations revealed the importance of pore-surface pockets, which utilize their size and electrostatic potential to smartly recognize the molecular sizes and quadruple moments of gas molecules to control their accessibility to the strongest adsorption sites.
Figures
Similar articles
-
Optimal Pore Chemistry in an Ultramicroporous Metal-Organic Framework for Benchmark Inverse CO2 /C2 H2 Separation.Angew Chem Int Ed Engl. 2021 Jul 26;60(31):17198-17204. doi: 10.1002/anie.202106769. Epub 2021 Jun 29. Angew Chem Int Ed Engl. 2021. PMID: 34043271
-
Sieving Effect for the Separation of C2 H2 /C2 H4 in an Ultrastable Ultramicroporous Zinc-Organic Framework.Chem Asian J. 2021 May 17;16(10):1233-1236. doi: 10.1002/asia.202100169. Epub 2021 Apr 26. Chem Asian J. 2021. PMID: 33844887
-
Controlling Pore Shape and Size of Interpenetrated Anion-Pillared Ultramicroporous Materials Enables Molecular Sieving of CO2 Combined with Ultrahigh Uptake Capacity.ACS Appl Mater Interfaces. 2018 May 16;10(19):16628-16635. doi: 10.1021/acsami.8b03358. Epub 2018 May 1. ACS Appl Mater Interfaces. 2018. PMID: 29671578
-
Electrostatically Driven Selective Adsorption of Carbon Dioxide over Acetylene in an Ultramicroporous Material.Angew Chem Int Ed Engl. 2021 Apr 19;60(17):9604-9609. doi: 10.1002/anie.202100584. Epub 2021 Mar 10. Angew Chem Int Ed Engl. 2021. PMID: 33524215 Free PMC article.
-
Ultramicroporous Building Units as a Path to Bi-microporous Metal-Organic Frameworks with High Acetylene Storage and Separation Performance.Angew Chem Int Ed Engl. 2019 Sep 16;58(38):13590-13595. doi: 10.1002/anie.201908378. Epub 2019 Aug 13. Angew Chem Int Ed Engl. 2019. PMID: 31407503 Review.
Cited by
-
Fine pore engineering in a series of isoreticular metal-organic frameworks for efficient C2H2/CO2 separation.Nat Commun. 2022 Jan 11;13(1):200. doi: 10.1038/s41467-021-27929-7. Nat Commun. 2022. PMID: 35017555 Free PMC article.
-
Molecular Sieving of Ethane from Ethylene through the Molecular Cross-Section Size Differentiation in Gallate-based Metal-Organic Frameworks.Angew Chem Int Ed Engl. 2018 Dec 3;57(49):16020-16025. doi: 10.1002/anie.201808716. Epub 2018 Nov 11. Angew Chem Int Ed Engl. 2018. PMID: 30304568 Free PMC article.
-
Deciphering Mechanisms of CO2-Selective Recognition over Acetylene within Porous Materials.Chem Bio Eng. 2024 May 13;1(5):366-380. doi: 10.1021/cbe.4c00035. eCollection 2024 Jun 27. Chem Bio Eng. 2024. PMID: 39975798 Free PMC article. Review.
-
Flue gas desulfurization and SO2 recovery within a flexible hydrogen-bonded organic framework.Nat Chem. 2025 May;17(5):727-733. doi: 10.1038/s41557-025-01744-9. Epub 2025 Feb 26. Nat Chem. 2025. PMID: 40011713
-
Controlling Thermal Expansion Behaviors of Fence-Like Metal-Organic Frameworks by Varying/Mixing Metal Ions.Front Chem. 2018 Jul 24;6:306. doi: 10.3389/fchem.2018.00306. eCollection 2018. Front Chem. 2018. PMID: 30137745 Free PMC article.
References
-
- Ariga K., Ito H., Hill J. P., Tsukube H. Chem. Soc. Rev. 2012;41:5800–5835. - PubMed
-
- Yu G., Jie K., Huang F. Chem. Rev. 2015;115:7240–7303. - PubMed
-
- Chen B., Xiang S., Qian G. Acc. Chem. Res. 2010;43:1115–1124. - PubMed
-
- Nugent P., Belmabkhout Y., Burd S. D., Cairns A. J., Luebke R., Forrest K., Pham T., Ma S., Space B., Wojtas L., Eddaoudi M., Zaworotko M. J. Nature. 2013;495:80–84. - PubMed
-
- Lin J.-M., He C.-T., Liu Y., Liao P.-Q., Zhou D.-D., Zhang J.-P., Chen X.-M. Angew. Chem., Int. Ed. 2016;128:4674–4678. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

