Understanding biological mechanisms underlying adverse birth outcomes in developing countries: protocol for a prospective cohort (AMANHI bio-banking) study
- PMID: 29163938
- PMCID: PMC5665675
- DOI: 10.7189/jogh.07.021202
Understanding biological mechanisms underlying adverse birth outcomes in developing countries: protocol for a prospective cohort (AMANHI bio-banking) study
Abstract
Objectives: The AMANHI study aims to seek for biomarkers as predictors of important pregnancy-related outcomes, and establish a biobank in developing countries for future research as new methods and technologies become available.
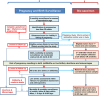
Methods: AMANHI is using harmonised protocols to enrol 3000 women in early pregnancies (8-19 weeks of gestation) for population-based follow-up in pregnancy up to 42 days postpartum in Bangladesh, Pakistan and Tanzania, with collection taking place between August 2014 and June 2016. Urine pregnancy tests will be used to confirm reported or suspected pregnancies for screening ultrasound by trained sonographers to accurately date the pregnancy. Trained study field workers will collect very detailed phenotypic and epidemiological data from the pregnant woman and her family at scheduled home visits during pregnancy (enrolment, 24-28 weeks, 32-36 weeks & 38+ weeks) and postpartum (days 0-6 or 42-60). Trained phlebotomists will collect maternal and umbilical blood samples, centrifuge and obtain aliquots of serum, plasma and the buffy coat for storage. They will also measure HbA1C and collect a dried spot sample of whole blood. Maternal urine samples will also be collected and stored, alongside placenta, umbilical cord tissue and membrane samples, which will both be frozen and prepared for histology examination. Maternal and newborn stool (for microbiota) as well as paternal and newborn saliva samples (for DNA extraction) will also be collected. All samples will be stored at -80°C in the biobank in each of the three sites. These samples will be linked to numerous epidemiological and phenotypic data with unique study identification numbers.
Importance of the study: AMANHI biobank proves that biobanking is feasible to implement in LMICs, but recognises that biobank creation is only the first step in addressing current global challenges.
Conflict of interest statement
Competing interests: The authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no competing interests. IR is the editor–in–chief of the Journal of Global Health. To ensure that any possible conflict of interest relevant to the journal has been addressed, this article was reviewed according to best practice guidelines of international editorial organizations.
Figures
References
-
- Wang H, Liddell CA, Coates MM, Mooney MD, Levitz CE, Schumacher AE, et al. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:957–79. doi: 10.1016/S0140-6736(14)60497-9. - DOI - PMC - PubMed
-
- World Health Organization. Maternal mortality fact sheet http://apps.who.int/iris/bitstream/10665/112318/1/WHO_RHR_14.06_eng.pdf?.... 2014.
-
- World Health Organization. Trends in maternal mortality 1990-2013 http://apps.who.int/iris/bitstream/10665/112682/2/9789241507226_eng.pdf?.... 2014.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials