Dexmedetomidine vs propofol for gastrointestinal endoscopy: A meta-analysis
- PMID: 29163971
- PMCID: PMC5676542
- DOI: 10.1177/2050640616688140
Dexmedetomidine vs propofol for gastrointestinal endoscopy: A meta-analysis
Abstract
Background and aim: Several randomized controlled trials have compared sedation with dexmedetomidine and propofol in gastrointestinal endoscopy, with contradictory results. We conducted a meta-analysis of data from randomized controlled trials that compared dexmedetomidine with propofol.
Methods: We searched PubMed, the Cochrane library, and the Igaku-chuo-zasshi database for randomized trials eligible for inclusion in our meta-analysis. We identified six eligible randomized trials from the database search, and compared the effect of propofol versus dexmedetomidine with respect to: (a) patient's satisfaction level, (b) body movement or gagging, (c) cardiopulmonary complications, and (d) change in heart rate. Data from eligible studies were combined to calculate pooled risk difference (RD) or weighted mean difference (WMD).
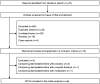
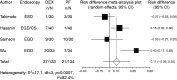
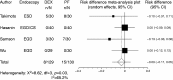
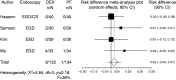
Results: Compared to propofol, dexmedetomidine significantly decreased the patient's satisfaction level (WMD: -0.678, 95% confidence interval (CI): -1.149 to -0.207, p = 0.0048), and there was no significant heterogeneity among the trial results. The pooled RD for developing body movement or gagging when using dexmedetomidine was 0.107 (95% CI: -0.09 to 0.305, p = 0.288), with no significant differences. Compared with propofol, the pooled RD for hypotension, hypoxia, and bradycardia with dexmedetomidine sedation were -0.029 (95% CI: -0.11 to 0.05), -0.080 (95% CI: -0.178 to 0.018), and 0.022 (95% CI: -0.027 to 0.07), respectively, with no significant differences. Compared to propofol, dexmedetomidine significantly decreased the heart rate (WMD: -10.41, 95% CI: -13.77 to -7.051, p ≤ 0.0001), without significant heterogeneity.
Conclusions: In gastrointestinal endoscopy, patient satisfaction level was higher in propofol administration, when compared to dexmedetomidine. The risk of complications was similar.
Keywords: Dexmedetomidine; endoscopy; meta-analysis; propofol; randomized controlled trial.
Figures



References
-
- Kashiwagi K, Hosoe N, Takahashi K, et al. Prospective, randomized, placebo-controlled trial evaluating the efficacy and safety of propofol sedation by anesthesiologists and gastroenterologist-led teams using computer-assisted personalized sedation during upper and lower gastrointestinal endoscopy. Dig Endosc 2016; 28: 657–664. - PubMed
-
- Nishizawa T, Suzuki H, Matsuzaki J, et al. Propofol versus traditional sedative agents for endoscopic submucosal dissection. Dig Endosc 2014; 26: 701–706. - PubMed
-
- Hashiguchi K, Matsunaga H, Hideyuki H, et al. Dexmedetomidine for sedation during upper gastrointestinal endoscopy. Dig Endosc 2012; 20: 178–183.
-
- Coursin DB, Maccioli GA. Dexmedetomidine. Curr Opin Crit Care 2001; 7: 221–226. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

