Efficacy and Safety of Methylprednisolone Sodium Succinate in Acute Spinal Cord Injury: A Systematic Review
- PMID: 29164020
- PMCID: PMC5684849
- DOI: 10.1177/2192568217706366
Efficacy and Safety of Methylprednisolone Sodium Succinate in Acute Spinal Cord Injury: A Systematic Review
Abstract
Study design: Systematic review and meta-analysis.
Objective: The objective of this study was to conduct a systematic review to assess the comparative effectiveness and safety of high-dose methylprednisolone sodium succinate (MPSS) versus no pharmacological treatment in patients with traumatic spinal cord injury (SCI).
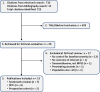
Methods: A systematic search was performed in PubMed and the Cochrane Collaboration Library for literature published between January 1956 and June 17, 2015. Included studies were critically appraised, and Grades of Recommendation Assessment, Development and Evaluation methods were used to determine the overall quality of evidence for primary outcomes. Previous systematic reviews on this topic were collated and evaluated using the Assessment of Multiple Systematic Reviews scoring system.
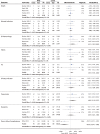
Results: The search yielded 723 citations, 13 of which satisfied inclusion criteria. Among these, 6 were primary research articles and 7 were previous systematic reviews. Based on the included research articles, there was moderate evidence that the 24-hour NASCIS II (National Acute Spinal Cord Injury Studies) MPSS regimen has no impact on long-term neurological recovery when all postinjury time points are considered. However, there is also moderate evidence that subjects receiving the same MPSS regimen within 8 hours of injury achieve an additional 3.2 points (95% confidence interval = 0.10 to 6.33; P = .04) of motor recovery compared with patients receiving placebo or no treatment.
Conclusion: Although safe to administer, a 24-hour NASCIS II MPSS regimen, when all postinjury time points are considered, has no impact on indices of long-term neurological recovery. When commenced within 8 hours of injury, however, a high-dose 24-hour regimen of MPSS confers a small positive benefit on long-term motor recovery and should be considered a treatment option for patients with SCI.
Keywords: MPSS; methylprednisolone sodium succinate; spinal cord injury; systematic review; traumatic spinal cord injury.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures







References
-
- Braughler J, Hall E. Effects of multi-dose methylprednisolone sodium succinate administration on injured cat spinal cord neurofilament degradation and energy metabolism. J Neurosurg. 1984;61:290–295. - PubMed
-
- Hall ED, Braughler JM. Glucocorticoid mechanisms in acute spinal cord injury: a review and therapeutic rationale. Surg Neurol. 1982;18:320–327. - PubMed
-
- Hall ED, Braughler JM. Effects of intravenous methylprednisolone on spinal cord lipid peroxidation and Na++K+)-ATPase activity. Dose-response analysis during 1st hour after contusion injury in the cat. J Neurosurg. 1982;57:247–253. - PubMed
-
- Akhtar AZ, Pippin JJ, Sandusky CB. Animal studies in spinal cord injury: a systematic review of methylprednisolone. Altern Lab Anim. 2009;37:43–62. - PubMed
-
- Eck JC, Nachtigall D, Humphreys SC, Hodges SD. Questionnaire survey of spine surgeons on the use of methylprednisolone for acute spinal cord injury. Spine (Phila Pa 1976). 2006;31:E250–E253. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

