Antiviral Activity of Itraconazole against Echovirus 30 Infection In Vitro
- PMID: 29164043
- PMCID: PMC5678193
- DOI: 10.24171/j.phrp.2017.8.5.05
Antiviral Activity of Itraconazole against Echovirus 30 Infection In Vitro
Abstract
Objectives: Echovirus 30 is a major cause of meningitis in children and adults. The aim of this study was to investigate whether the antifungal drug itraconazole could exhibit antiviral activity against echovirus 30.
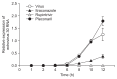
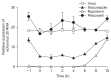
Methods: The cytopathic effect and viral RNA levels were assessed in RD cells as indicators of viral replication. The effects of itraconazole were compared to those of two known antiviral drugs, rupintrivir and pleconaril. The time course and time-of-addition assays were used to approximate the time at which itraconazole exerts its activity in the viral cycle.
Results: Itraconazole and rupintrivir demonstrated the greatest potency against echovirus 30, demonstrating concentration-dependent activity, whereas pleconaril showed no antiviral activity. Itraconazole did not directly inactivate echovirus 30 particles or impede viral uptake into RD cells, but did affect the initial stages of echovirus 30 infection through interference with viral replication.
Conclusion: Itraconazole can be considered a lead candidate for the development of antiviral drugs against echovirus 30 that may be used during the early stages of echovirus 30 replication.
Keywords: itraconazole; meningitis; viruses.
Conflict of interest statement
CONFLICTS OF INTEREST No potential conflict of interest relevant to this article was reported.
Figures


References
-
- Rudolph H, Prieto Dernbach R, Walka M, et al. Comparison of clinical and laboratory characteristics during two major paediatric meningitis outbreaks of echovirus 30 and other non-polio enteroviruses in Germany in 2008 and 2013. Eur J Clin Microbiol Infect Dis. 2017 doi: 10.1007/s10096-017-2979-7. In press. https://doi.org/10.1007/s10096-017-2979-7. - DOI - PubMed
-
- Rudolph H, Schroten H, Tenenbaum T. Enterovirus infections of the central nervous system in children: an update. Pediatr Infect Dis J. 2016;35:567–9. doi: 10.1097/INF.0000000000001090. https://doi.org/10.1097/INF.0000000000001090. - DOI - PubMed
-
- Wieczorek M, Krzysztoszek A, Figas A. Molecular characterization of echovirus 30 isolates from Poland, 1995–2015. Virus Genes. 2016;52:400–4. doi: 10.1007/s11262-016-1310-5. https://doi.org/10.1007/s11262-016-1310-5. - DOI - PubMed
-
- Österback R, Kalliokoski T, Lähdesmäki T, et al. Echovirus 30 meningitis epidemic followed by an outbreak-specific RT-qPCR. J Clin Virol. 2015;69:7–11. doi: 10.1016/j.jcv.2015.05.012. https://doi.org/10.1016/j.jcv.2015.05.012. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

