Development of Novel Patient-Derived Xenografts from Breast Cancer Brain Metastases
- PMID: 29164052
- PMCID: PMC5673842
- DOI: 10.3389/fonc.2017.00252
Development of Novel Patient-Derived Xenografts from Breast Cancer Brain Metastases
Abstract
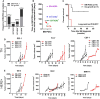
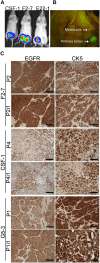
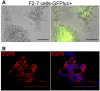
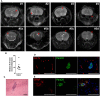
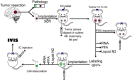
Brain metastases are an increasing burden among breast cancer patients, particularly for those with HER2+ and triple negative (TN) subtypes. Mechanistic insight into the pathophysiology of brain metastases and preclinical validation of therapies has relied almost exclusively on intracardiac injection of brain-homing cells derived from highly aggressive TN MDA-MB-231 and HER2+ BT474 breast cancer cell lines. Yet, these well characterized models are far from representing the tumor heterogeneity observed clinically and, due to their fast progression in vivo, their suitability to validate therapies for established brain metastasis remains limited. The goal of this study was to develop and characterize novel human brain metastasis breast cancer patient-derived xenografts (BM-PDXs) to study the biology of brain metastasis and to serve as tools for testing novel therapeutic approaches. We obtained freshly resected brain metastases from consenting donors with breast cancer. Tissue was immediately implanted in the mammary fat pad of female immunocompromised mice and expanded as BM-PDXs. Brain metastases from 3/4 (75%) TN, 1/1 (100%) estrogen receptor positive (ER+), and 5/9 (55.5%) HER2+ clinical subtypes were established as transplantable BM-PDXs. To facilitate tracking of metastatic dissemination using BM-PDXs, we labeled PDX-dissociated cells with EGFP-luciferase followed by reimplantation in mice, and generated a BM-derived cell line (F2-7). Immunohistologic analyses demonstrated that parental and labeled BM-PDXs retained expression of critical clinical markers such as ER, progesterone receptor, epidermal growth factor receptor, HER2, and the basal cell marker cytokeratin 5. Similarly, RNA sequencing analysis showed clustering of parental, labeled BM-PDXs and their corresponding cell line derivative. Intracardiac injection of dissociated cells from BM-E22-1, resulted in magnetic resonance imaging-detectable macrometastases in 4/8 (50%) and micrometastases (8/8) (100%) mice, suggesting that BM-PDXs remain capable of colonizing the brain at high frequencies. Brain metastases developed 8-12 weeks after ic injection, located to the brain parenchyma, grew around blood vessels, and elicited astroglia activation characteristic of breast cancer brain metastasis. These novel BM-PDXs represent heterogeneous and clinically relevant models to study mechanisms of brain metastatic colonization, with the added benefit of a slower progression rate that makes them suitable for preclinical testing of drugs in therapeutic settings.
Keywords: HER2+; brain colonization; brain metastases models; breast cancer; patient-derived xenograft; triple negative.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

